The Principles of Photography
The development of photography has had a profound effect on society. The Civil War in the United States became one of the first historical events to be chronicled by photography as have all major historical events since that time. Portraits became accessible to socioeconomic classes in ways not possible when the primary means of portraiture was to hire an artist to draw or paint the subject. It has been argued that one of the most profound effects that the introduction of photography had on Art was to free up the artist to no longer be constrained as the conveyor of reality - rather the artist was now free to interpret a subject as he or she saw fit. Theis led inevitably to the impressionist movement and to ever increasing abstraction and surrealism. But photography has been more than a method of recording events and people. The limits of the photographic processes have been pushed to achieve a wide variety of artistic effects. Photos can be overexposed or underexposed, multiple images can be created and the color values changed through chemical processes.
The word photography comes from the combination of the Greek words drawing and light, and signifies the creation of an image with light. The camera obscura had been known for centuries and was employed by artist to help transfer projected images into drawings, but it wasn't until the early 1800s that cameras began to be used for focussing light onto photosensitive chemical compounds so that the images could be recorded. It is those chemical processes that will be the focus this page. Digital photography is becoming an increasingly important medium, and it also relies on light reflecting off of an object to capture its image, but that process is distinctly different, involving the focusing of light onto a detector that creates an array of electrical signals from the received light and records those signals as intensities and wavelengths in digital form. While film photography is being replaced by the digital realm, film photography and classical photographic printmaking still offers the artist a medium that has tremendous flexibility and opportunities for creativity.
In order for photography to work, the light reflecting from an scene must be focused on photosensitive substance that consequently undergoes a chemical reaction in proportion to the amount of light that strikes it. This overall process falls under the category of photochemistry, and shining light on chemical substances is a routine technique by which chemists add energy to a system to make a reaction occur. Photochemical processes can be very different from the reactions that occur by simply heating a reaction mixture and are dependent on the wavelength of light employed. The earliest photographic images were created with a single color process that, for simplicity, we will label as black and white photography, although many of he early images will be distinctly brown or perhaps blue. These photographs can be modified by chemical means to change the color of the image in a a process called toning that we will discuss later once the fundamentals are described. True color photography is a complicated technology but it still relies on the same fundamental photochemical reaction. It will be described last.
Single Color Photography
Photography involves several distinct steps. In the earliest days, the photographer had not only to make and process an image but also had to create the photographic papers and plates to be used. Once these materials were in hand, the taking of a photographic image could be divided roughly into four steps, although not all steps were used in all processes. The basic steps are outlined below, and this will be followed by some information on variations in the process.
- Exposure
- Development
- Stopping
- Fixing
- Washing
Exposure: the fundamental chemical process
There are a variety of chemical compounds that are photosensitive, but the most widely used from the earliest days of photography has been the conversion of silver halides, AgX (X = Cl, Br, I), to produce metallic Ag and X2. This phenomenon can be easily observed — silver halides are prepared they are generally colorless/white or light yellow solids that will discolor if not protected from light, and the reduction process is easier the heaveir the halide is. Consequently, silver halides are stored in dark brown bottles to reduce unwanted exposure to light. Upon initial exposure of the silver halides, whether in a photographic paper, on a photographic plate or in a photographic film, a few silver atoms are produced in a reduction process. The different silver halides have different sensitivities and tuning the relative ratios of the different halides can tune the sensitivity of the film or paper. Please note that the mixed halide compounds are not simply mixtures of the individual silver halides. They are better written, for example, as AgCl1-xBrx. This formulation indicates that the halide ions are randomly distributed throughout the solid particle, as opposed to being a mixture of AgCl and AgBr particles. The different halides also have different responses to development and fixing which creates additional ways of modifying the photographic process. The particle size of the silver halide compound is also important in determining rates of reaction as well as ultimate resolution. Some other photosensitive materials will be encountered as specific types of processes are described below.
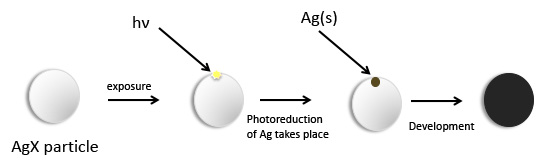
Development
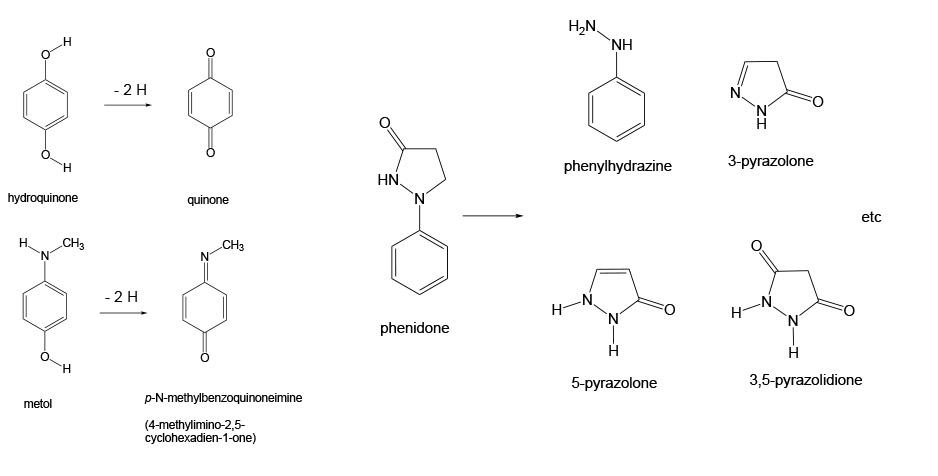
It would be very hard to detect the initial image with the naked eye. In order to produce the recognizable image, a development process must occur. In this process a reductant is added to reduce more of the silver halide. The key point of this development is that the reduction process is normally slow but it can be catalyzed to occur at a reasonable rate, and, as it turns out, those initially produced silver atoms are good catalysts for the reduction reaction. Some simple organic compounds are good developing agents. These include hydroquinone, metol and phenidone, shown below. The oxidation of phenidone leads to a mixture of products.
Stopping
Once the development process has gone far enough to create a good quality image, the reduction process needs to stopped, or else the entire image will become black. The reduction process is senstive to the pH of the solution. It is very slow in acidic solution, and acetic acid is commonly used as the stopper. Acetic acid, CH3CO2H is the active ingredient in vinegar and the oxidation product of ethanol, CH3CH2OH. A photographic paper or film is simply placed in a bath containing the acid for a specified time and then rinsed with water once the reaction has been stopped to remove the excess acid.
Fixing
After stopping the reaction, any residual silver halides need to be removed as they will remain subject to photoreaction with light. They would ultimately darken and degrade the image. This process is known as fixing. The photographic film or paper is then treated with a solution of sodium thiosulfate, Na2S2O3, also known as sodium hyposulfite (also simply called hypo), which reacts with the residual silver halides to form a soluble complex as shown in the equation below. Silver is classified as a 'soft' metal and has a strong affinity to sulfur-based ligands such as the thiosulfate ion. Simularly, metallic silver is also subject to reaction with sulfur compounds in the atmosphere such as H2S, forming Ag2S(s). Silver sulfide is a brown compound and is what is formed when silver objects tarnish. This tendency to react with sulfur, as well as selenium which lies in the same group of the periodic table, is used to tone photographic prints, but nonintentional reaction with atmospheric sulfur compounds will degrade photographs over time. Consequently, silver prints need to be carefully stored to reduced these unwanted reactions.
Washing
After fixing, the film or print is washed thoroughly to remove excess chemicals. With time, remaining compounds can discolor, compromising print and negative quality. The wash is not a substitute for fixer, as no amount of washing can effectively remove the insoluble silver halides.
Variations on the Photographic Process
Negative and Positive Images

When light directly strikes a photosensitive paper or film, the image created is a negative image of the actual scene - the light areas in the subject will be dark and the dark areas light. As will be seen, in some cases, placing a dark background behind a negative image will make it appear to be a positive, but the process quickly evolved such that the process of making a photographic print involved shining light through a negative image onto a piece of photosensitive paper to reverse the negative image and produce a positive. When carrying out this print process, the negative can be placed directly onto the photosensitive paper and then irradiated. The result is known as a contact print because the negative and the resultant image were in direct contact with each other. Alternatively the negative can be placed in an enlarger that allows the negative to be placed above the paper and lenses will focus an enlarged image onto the paper. There are limits to which photographic negatives can be enlarged as the increased size will be accompanied by a reduction in resolution with the resulting image appearing grainy.
Platinum and Palladium vs Silver
Silver is an easily reduced metal (reduction potential is +0.80 V), but palladium (+0.915 V), platinum (+1.2 V) and gold (+1.5 V) are even easier to reduce (and more difficult to oxidize). In the early days of photography, platinum compounds were also explored as possible photoactive materials, but platinum was found to be less sensitive than silver to light. This problem was overcome by the use of a sensitizer. Ferric oxalate, Fe2(C2O4)3, was found to sensitize potassium tetrachloroplatinate, K2PtCl4, to reduction by light. Ferric oxalate is reduced to ferrous oxalate, Fe(C2O4), upon irradiation, and the ferrous oxalate then reduces the Pt2+ to Pt0. Platinum is more expensive than palladium, and palladium, or a mixture of palladium and platinum, is often used in place of pure platinum. The process developed in the late 1800s when platinum was relatively inexpensive, but its use during World War I led to a dramatic price increase and platinum prints went out of fashion.
In addition to producing platinum or palladium prints by direct reduction of platinum or palladium salts, it is also possible to create a platinum or palladium print by starting with a silver print and using the appropriate Pt or Pd salts to electrochemically replace some of the silver in the print. See the section on Toning below.
In contrast to silver prints, the platinum (or palladium) sits on the surface of the paper rather than being embedded in it. Platinum prints have a larger tonal range than silver prints and also will not fade over time, since platinum is much more resistant to oxidation than silver and the prints are considered archival quality. Palladium prints are also long-lived and are of archival quality, although they don't have quite the stability as platinum. It is estimated that platinum prints could last thousands of years. Platinum prints are usually made by contact printing from a silver-based negative.
Creating Permanent Images
Even though the camera obscura had been known from the middle ages, a successful way to produce a permanent print took some time to discover and it wasn't until the fundmentals of chemistry were beginning to be refined into a discipline – around the beginning of the 1800s – that the photograghic process as we now know it was created. in the early 1800s, Joseph Nicéphore Niépce was able to produce to the first permanent photographic image by using a camera obscura to focus and image on a bitumen coated plate. Niépce had noted that bitumen that had been exposed to light hardened – what we now know to be the result of polymerization of the organic molecules in the bitumen – and was no longer susceptible to being removed with solvents. He had already created images on salted paper - paper embedded with silver chloride - but those images would fade with time, so the bitumen process produced the first known permanent photographic images. After exposure of a bitumen-coated plate to a focused image, the plate was rinsed with lavendar oil to remove the still-soluble bitument. This plate could then be etched by conventional chemical methods and used in a normal printing press to produce paper prints of the photoengraved image. Niépce collaborated with Louis-Jacques-Mandé Daguerre, but Niépce died in 1833 and Daguerre went on to use the basics to create the daguerreotype process.
Salted Paper Print

Salted paper prints were first created by William Henry Fox Talbot in the 1830s. Talbot made a photosensitive paper by treating the paper with a solution of sodium chloride and then a solution of silver nitrate, which results in the formation of insoluble silver chloride. This process creates photoactive silver chloride particles embedded in the paper.
After exposure, the paper was treated with a concentrated salt solution, such as sodium bromide, which would render the paper much less photosensitive. In 1839, it was discovered that the salt sodium thiosulfate (hypo) was effective at making the prints even more stable. We now know that this results from dissolution of the excess silver halides, allowing them to be washed away but does not affect the reduced metallic silver. In the salted paper print method, the paper is exposed long enough to create a clear image and consequently exposure times were long, but Talbot refined the technology by using silver iodide, which required much shorter exposure times but reequired an additional chemical treatment step to develop the image. This improved technology was known as the calotype, or talbotype, process described next.
Calotype, or Talbotype, Process

The calotype process was developed by William Henry Fox Talbot in 1841. It involved first creating a negative image using a thin paper that had been imbedded with silver nitrate and potssium iodide. The silver iodide paper was found to be superior for creating a negative image as compared to the silver chloride paper that Talbot had developed in 1835. It allowed for faster exposure times and introduced the developing stage by treating the exposed iodized paper with a solution of silver nitrate, acetic acid and gallic acid. Once this negative was made, it could be used to prepare several positive prints using silver chloride-based salted paper.
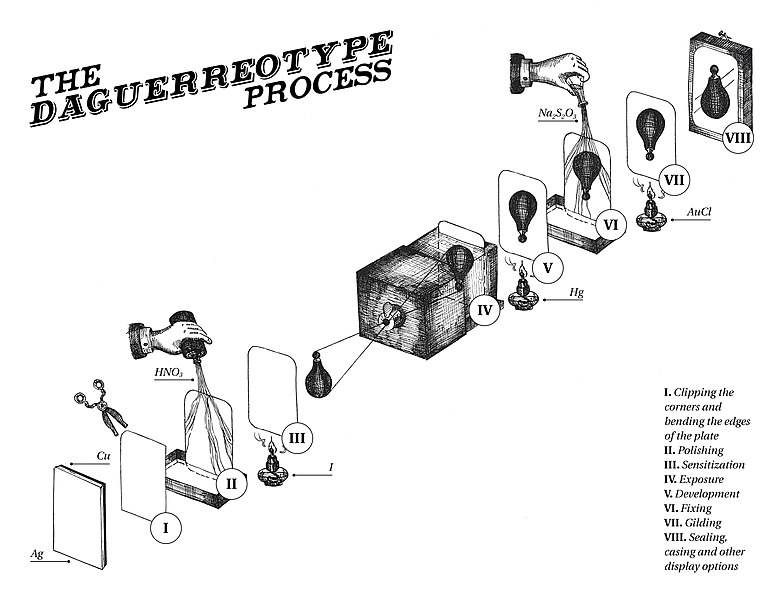
Daguerreotypes

Some of the earliest photographs are daguerreotypes, a process invented named after Louis-Jacques-Mandé Daguerre around 1837. Copper metal is plated with a thin layer of silver, often by mechanical rolling and heating a thin silver foil on top of the copper. The silver is then sensitized by reaction with iodine, I2, or the other halogens to produce a silver iodide coated surface. elemental iodine is a shiny dark solid that sublimes to give purple iodine gas. After exposure, daguerreotypes were developed by placing the exposed image in a box filled with mercury vapor, consequently the process is quite toxic. Production of modern daguerreotypes would require specialized facilities to avoid exposure to mercury vapors. Daguerreotypes are fragile and the silver film flakes off easily from the surface. The stability of the film was enhanced by treating the image with gold chloride under mild heating. The gold will replace a small amount of the silver in the image by electrochemical replacement and the added gold will make the image warmer in tone. It is difficult to get images of live objects with daguerreotypes because the exposure times needed to get a good image are long, and the subject needs to remain still for a relatively long period of time or else the image will be blurry. In some classic daguerreotypes of cityscapes, moving vehicles are seen only as blurred lines in the images. Nevertheless, the resolution in daguerreotypes can be very high, showing amazing levels of details.
Collodion Process or Collodion Wet Plate Process

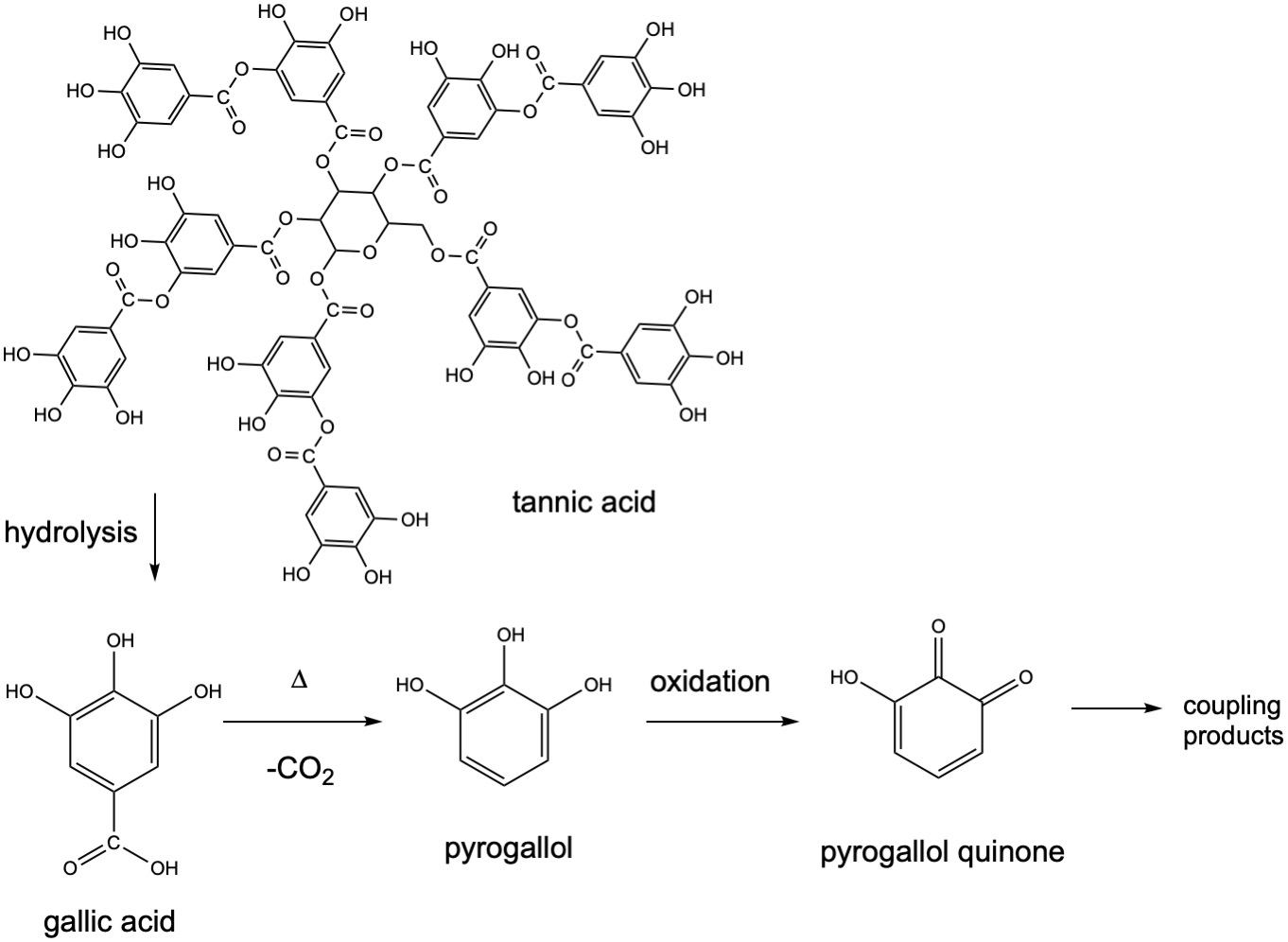
In the collodion process, or the "collodion wet plate process", the silver halide material is created in collodion, which is cellulose nitrate. The photographic material is then coated onto a glass substrate, sensitized, exposed and developed with pyrogallol (1,2,3-trihydroxybenzene) within about fifteen minutes, necessitating the availability of a nearby darkroom. The process produces a negative image on a glass support. This was an improvement over the calotype process, discovered by Henry Fox Talbot (paper negatives), and the daguerreotype that made a one-off positive image and could not be duplicated. The pyrogallol is obtained from tannins. When tannic acid is hydrolyzed, it produces gallic acid which in turn loses CO2 upon heating to yield pyrogallol. Pyrogallol is oxidized to a pyrogallol quinone, which can undergo further coupling reactions to give more complex products.
Ambrotype

Ambrotypes are a variation on the collodion wet-plate process. An image is produced by coating a piece of glass with the collodion emulsion that contains iodide ions, which is then placing it in a solution of silver nitrate. This results in the formation of silver iodide crystals inside the collodion emulsion. The plate must be kept wet, then placed in a camera, exposed and developed quickly. When viewed against a dark background, the image appears a a positive.
Tintype (Melainotype or Ferrotype)

Surprisingly, no tin is involved in the production of a tintype. Instead, the image is created similarly to an ambrotype but, instead of glass, the photosensitive emulsion is placed on an iron substrate with a dark lacquer coating. A direct positive image is produced.
Albumen Print

Albumen prints, also known as albumen silver prints, are created on a photosensitive paper prepared by first coating the paper with albumen mixed with sodium or ammonium chloride. Albumen is the protein found in egg whites. After drying, the paper is exposed to silver nitrate, which results in the formation of small grains of silver chloride inside the albumen matrix. The albumen-coated paper is then exposed to sunlight or ultraviolet light through a negative, which was historically created on a glass plate, to produce the final print. Once the exposure is completed, the print is fixed using sodium thiosulfate as described above. Albumen prints may be toned
Cyanotypes

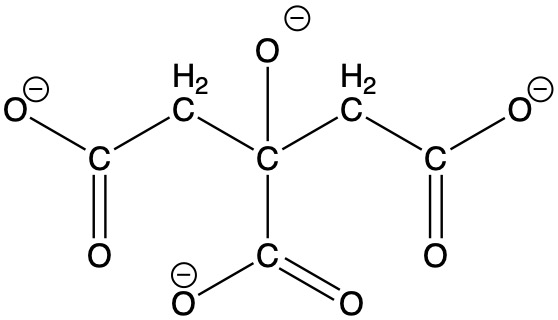
Cyanotypes are known for their characteristic blue-tint and were commonly used for creating blueprints.Unlike the other prints described here, cyanotypes are not based on silver halides. Instead, the process employs potassium ferricyanide, K4[Fe(CN)6], and ferric ammonium citrate, [NH4]5[Fe(C6H4O7)2]·2H2O. In the ferric ammonium citrate, each Fe3+ ion is bound to two citrate4- ions through the oxygen atoms of the three carboxylate groups as well as the oxygen atom of the alcohol function (see structure below). Potassium dichromate, K2Cr2O7, can be added to enhance the contrast in the final print. A solution of these chemicals is prepared and applied to paper, but images can also be made on cloth or any other surface that will absorb the solution. The resulting paper is then exposed to sunlight or some other ultraviolet light source through a negative to produce a positive print. It has been quite popular for school projects to create prints of leaves and other common objects by placing them directly on the sensitized paper in bright sunlight. The ultraviolet light causes a complex chemical reaction resulting in the formation of Fe4[Fe(CN)6]3, known as Prussian blue (see Inorganic Pigments for the structure of Prussian blue). Similar chemistry is used to tone photographs (see below).
Carbon Print

Carbon prints are similar to cyanotype in that they do not rely on the reduction of silver halides to produce the photographic image. In this method, a pigment is dispersed in a layer of gelatin on a thin layer of paper. Originally the pigment was carbon black and hence the name given to the papers was carbon tissue. Carbon black will produce black and white images, but other monotone images such as sepia were also produced. A wide variety of pigments can, however be used and the method can produce, with some considerable skill, full color prints by utilizing three layers of the tissue containing cyan, magneta and yellow pigments. Before use, the tissue is sensitized with potassium dichromate, which is also used to enhance cyanotype images. Once exposed to light, the exposed areas of gelatin become hardened and are no longer soluble in water. The unreacted gelatin is washed away, and the thin, hardened gelatin layer is transferred to a support paper. In the case of color prints, each of the three colored tissue sheets must be exposed and processed separately. These are then applied carefully in exact register onto the support paper to produce the final full color images. In some cases, a black layer is also added on top of these to help define the edges of the subjects in the paper. The result can be a very stable, long-life print of high quality. The transfer technique also means that the print exhibits a slight relief profile on top of the support paper.
Toning a Photography
Once a photographic print has been made the tones of the print can be modified by various chemical reactions. These reactions make use of the reactivity of the silver particles, which are responsible for the image, with various types of chemical reagents. Quite simply, the photograph to be toned is immersed in a bath of the appropriate chemical reagents for a short period of time and then rinsed to remove excess chemicals and stop the toning process. The degree of toning is controlled by the concentration of the reagents, the length of toning time and the temperature. Here are some common examples:
Sulfur and Selenium toning
For sepia toning, a print is first treated with potassium ferricyanide to oxidize the silver particles in the print. The resulting silver halide is then treated with sulfur-based reagents such as sodium thiosulfate or thiourea to produce silver sulfide, Ag2S. Sodium thiosulfate in the presence of acid, produces sulfur that then reacts with the oxidized silver producing the brown color known as sepia. Sepia-toning improves the archival stability of photographs as coating the silver particles with silver sulfide makes them more inert. Similarly, selenium treatment results in the production of Ag2Se, which has a red-brown color if used in dilute solution and a purple-brown color when concentrated. The effect also varies with the paper used. AgCl/AgBr mixed papers react very strongly but pure AgBr emulsions are affected only slightly.


Potassium ferricyanide toning
Potassium ferricyanide, K3[Fe(CN)6] reacts with a small amount of the Ag(s) to form Fe4[Fe(CN)6]3, which we have encountered in inorganic pigments as Prussian blue. The oxidation state of iron in K3[Fe(CN)6] is +3, and the reaction with silver reduces some of the iron to Fe2+ to give a mixed valence compound that has a bright blue color. To emphasize the oxidation states of the metals, the formula of this product can be written as (Fe3+)4([Fe2+(CN)6]4-)3. The silver is oxidized to Ag+.
Gold, Platinum and Palladium Toning
Gold toning takes advantage of the different reduction potentials of gold and silver. Since gold is more difficult to oxidize, and consequently more easily reduced, solutions of gold chloride will displace silver metal as soluble oxidized silver particles while simultaneously producing metallic gold particles. Gold-toned photographs are known for the blue-black tone; however, other shades can be produced, and gold can be used in conjunction with a sepia toner to produce an orangish red tone. The same methodology can be used to tone with platinum and palladium since both of these metals also have reduction potentials that are greater than than of Ag+, (+1.2V and +0.915V, respectively). The toning process should not be confused with the platinum and palladium print processes described above, in which the metals are reduced directly without going through an intermediate silver print.
Au3+(aq) + 3e- → Au(s) |
E = +1.52 V |
Ag+(aq) + e- → Ag(s) |
E = +0.80 V |
Au3+(aq) + 3 Ag(s) → Au(s) + 3Ag+(aq) |
E = +0.72 V |
Color Photography
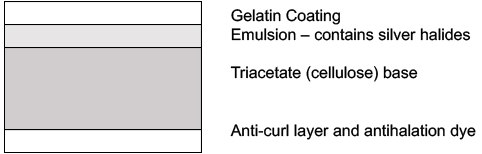
As already described for carbon prints above, full color images could be prepared by making separate, thin layers of pigmented images using the hardened gelatin method. This was a very labor-intensive process and accessible only to the afficianado. Much simpler color photography was developed from the mid 1900s using the same basic principles, but incorporating the three colors into separate layers within a piece of photgraphic film or paper. Color film required three layers that correspond to additive color mixing of light - red, green and blue. In the original process developed by Eastman Kodak, the film was first developed as silver images with the layers then being altered by use of dyes to produce three different colors within the layers. Once developed, the silver was removed, leaving only the dyes in the layers. Agfa, a German firm, advanced the technology by incorporating the coupling dyes directly into the film. Similarly, color photographic papers are made adding separate color layers onto the support paper. The ordering of the layers is arranged according to how well the light of that particular color penetrates into the film or paper.

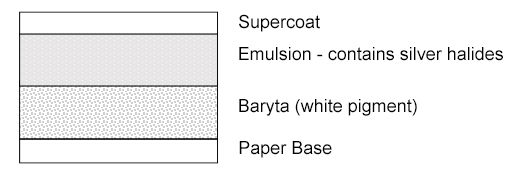
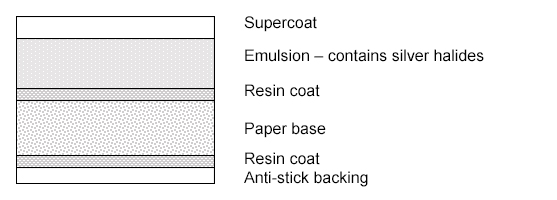
Identification of Photographs
The first steps in identification of a photography rely on a visual inspection of the object, which can be aided tremendously by magnification. Much can be learned by simply observing the nature of the support material, the surface of the photograph and the ways in which a photograph is mounted. Some examples of things to look for can be found at 5.2.1 Types of Photographs, part 1: 19th and Early 20th Century, G. Albright and M. Fischer, Northeast Document Conservation Center (NEDCC) and Beginner's Guide To Identifying Different Forms of Antique Photographs, Antique Photos, Youtube. Daguerreotypes are particularly characteristic by observing the metallic sheen of the image as it is viewed from various directions. Tin-types, which were created on an iron support, are magnetic and can simply be tested by placing a magnet on the back of the print.
Shortcuts
Resources and References
- The oxidation of metol (N-methyl-p-aminophenol) in aqueous solution by UV/H2O2 photolysis, Andreozzi R, Caprio V, Insola A, Marotta R, Water Research, 34, 2000, 463-472, https://doi.org/10.1016/S0043-1354(99)00183-9.
- Daguerreotype, Wikipedia.
- Calotype, Wikipedia.
- Carbon Print, Wikipedia.
- Synthesis, Spectroscopic and Structural Characterization of the First Mononuclear, Water Soluble Iron−Citrate Complex, (NH4)5Fe(C6H4O7)2·2H2O,Matzapetakis M, Raptopoulou, C P, Tsohos A, Papaefthymiou V, Moon N, Salifoglou A, J. Am. Chem. Soc. 1998, 120, 50, 13266–13267, https://doi.org/10.1021/ja9807035.
Crystal Structure Data
- Structures for which crystallographic information format (CIF) were available were obtained from the Cambridge Crystallographic Data Centre. Those not in the database were created using ChemDoodle 3D and/or CrystalMaker.
- The crystal structure of K2PtCl4 and K2PdCl4 with estimates of the factors affecting accuracy, Mais R.H.B., Owston P.G., Wood A.M., Acta Crystallographica, 1972, 28, 393-399.
- Unraveling the structure of iron(III) oxalate tetrahydrate and its reversible Li insertion capability, Ahouari H, Rousse G, Rodriguez-Carvajal J, Sougrati M-T, Saubanere, M,. Courty M, Recham, N, Tarascon, J-M, Chemistry of Materials, 2015, 27, 1631-1639.
- The structure of oxalite FeC2O4·2(H2O), Mazzi F, Garavelli C, Periodico di Mineralogia, 1957, 26, 269-303.
- Synthesis, Spectroscopic and Structural Characterization of the First Mononuclear, Water Soluble Iron−Citrate Complex, (NH4)5Fe(C6H4O7)2·2H2O, Matzapetakis, M, Raptopoulou, C P, Tsohos A, Papaefthymiou, V, Moon, N. A.Salifoglou, Journal of the American Chemical Society, 1999, 120, 13266-13267, DOI: 10.5517/cc3trc0
Credits for the Header Image
- Seated Couple, W. A. Degele, 1850s, Daguerreotype in passe-partout mount, from the Collection of the Museum of Fine Arts Houston, Public Domain.
- Apollo 11 Lunar Lander Neil Armstrong, Public domain, via Wikimedia Commons
- Major-General Joseph Hooker, Mathew B. Brady, c. 1863, Salted paper print from glass ngative with applied color, from the collection of the Museum of Fine Arts Houston, Public Domain.
Department of Chemistry

Houston, TX
6100 Main St., Houston, TX 77005-1827 | Mailing Address: P.O. Box 1892, Houston, TX 77251-1892 713-348-0000 |












