Inorganic minerals have served as source of pigments and the origin of their colors is discussed at Pigments, Dyes, Inks and Glazes. On this page, information is presented about the most used inorganic pigments coming from both naturally-occurring mineral forms as well as synthetic compounds.
Minerals as Pigments
Cave paintings employed common compounds such as iron oxide minerals in addition to charcoal for black and kaolin clay, calcite (the major componenet of chalk) or gypsum for white. There are several oxides of iron that can be used for pigments. Hematite, Fe2O3, which produces red colors, limonite, FeO(OH)·(H2O)n, yielding yellow to brown colors, and magnetite, Fe3O4, which is black. While both hematite and magnetite form crystalline materials, limonite is amorphous. Charcoal results from the partial burning of wood and other suitable plants. While the carbon structures present in charcoal are a complex mixture, they are based largely on graphite and related graphitic structures. Because the hydrogen has largely been removed from the organic matter from which charcoal is derived, charcoal itself is considered an inorganic substance. Kaolinite is a white clay that is low in iron impurities, which would tend to make the clay red. In addition to being used as a pigment, it is also important in the manufacure of fine porcelain.
Hematite, Fe2O3
Magnetite, Fe3O4
Carbon, C
Al2Si2O9
Calcium carbonate, CaCO3
Calcium sulfate dihydate, CaSO4·2H2O
A number of naturally-occurring metal carbonates and metal oxides have been used as pigments. Examples include the carbonates of calcium and lead and the oxides of titanium, lead and zinc. Conservation problems for paintings that contain the lead and zinc compounds have proven to be particularly problematic owing to the formation of lead and zinc soaps, which have a nasty habit of flaking off of the canvas. The chemistry of this process is discussed on the Paintings page. Titanium white is found in nature as the mineral rutile but was not used extensively until after 1920 because of difficulties in processing the mineral. Unlike the other whites, when mixed with drying oils titanium dioxide, also known as titania, dries to a spongey mass rather than a nice film, so TiO2 must be mixed with zinc white or baryte in order to form a usable oil paint. If the fraction of zinc white is large, the paint will be known as titanium zinc white. Titanium dioxide is the major white pigment used in house paints, having replaced lead pigments as a non-toxic alternative. Barium sulfate is also a common white pigment that is found in nature as the mineral baryte. Barium sulfate finds use in medical imaging of the GI tract because of barium has a high absorption for X-rays and also has low toxicity due to its low solubility.
In addition to kaolinite, a variety of white pigments that have been used throughout time, but if you have tried to pick out a white paint at the hardware or art store, you will realize that not all whites are the same. That is very true of the white pigments listed here. They have slightly varying colors, varying degrees of opacity and cost that make some more useful for particular applications. For example, calcite is inexpensive and routinely used in gesso, the ground layer in many paintings. Titanium white has the advantage of being very stable, compared to zinc and lead whites that may produce soaps under some conditions (see Pigments, Dyes, Inks and Glazes.
Barium sulfate, BaSO4
Lead white, basic lead carbonate (idealized structure), 2PbCO3·Pb(OH)2
Rutile, TiO2
TiO2, Anatase
ZnO
Azurite and Malachhite: Azurite, Cu3(CO3)2(OH)2, and malachite, Cu2(CO3)(OH)2, are related carbonate minerals that have blue and green colors, respectively. Azurite converts into malachite upon weathering in nature and results from the loss of CO2 from the azurite. Their structures are related to that of synthetic verdigris, in which acetate ions replace carbonate. Azurite will convert into malachite upon loss of CO2.

Cu3(CO3)2(OH)2
Cu2(CO3)(OH)2
Realgar and Orpiment: Realgar and orpiment are two arsenic-sulfide pigments that were known to the ancient Egyptians. Because they contain arsenic, they are toxic, but this was not appreciated by the Egyptians who used them in cosmetics.
As4S4
As2S3
Cinnabar: Is a naturally-occuring mercuric sulfide, HgS, that was used as a red pigment. The Romans mined cinnabar in Spain in the a region near the border with Portugal. While mineral deposits are available, the Chinese developed a simple synthesis from elemental Hg(l) in direct reaction with sulfur. The pigment is highly toxic because of the mercury, and so other red pigments have replaced it.

Mercuric sulfide, Cinnabar, HgS
Lapis Lazuli: Lazurite, the mineral name of lapis lazuli, has the formula (Na,Ca)8Al6Si6O24(S,SO4). It is a brilliant blue that was known in painting as ultramarine. The color arises from the presence of the S3- radical ion trapped in the crystal lattice. It was very expensive and artists in the Middle Ages and later would sometimes scavenge the blue pigments from paintings to use in new works. It was especially desirable for use in the robes of religious figures, most notably the Virgin Mary in Christian paintings from the Middle Ages and Renaissance. In the 1800s a synthetic version was created known as French Ultramarine Blue. As a result of the discovery and the expense of the natural mineral, lapis lazuli dropped out of favor as a pigment and was no longer referred to as ultramarine. It may still be purchased from specialty painting supply stores.

Lazurite, (Na,Ca)8Al6Si6O24(S,SO4, Cl)
Celadonite/Glauconite: Celadonite and glauconite are closely related minerals that have a greenish white to green color. The pigment form is known as green earth that has been used for centuries. The Koreans are well-known for the creation of green celadonite pottery.

Celadonite or Glauconite, K(Mg,Fe2+)(Fe3+,Al)2Si4O12
Synthetic Inorganic Pigments
From ancient times, advanced cultures have developed ways to make pigments whose natural mineral sources were rare and expensive. This search for alternatives continued from the Egyptians and Chinese through the Middle Ages and into modern times. Identification of which pigments were used in an object can help to establish the time period in which it was, or wasn't, made. Some caution must be used though as later owners and conservators sometimes touched up older paintings with newer pigments. Nevertheless, we don't normally expect Egyptian painted objects to contain Prussian blue or cadmium yellow.
We should not think that the the ancient civilizations were completely devoid of science and technology. Our historic times periods of the stone age, copper age, bronze age and iron age attests to the development of metallurgical sciences with the spin offs that come from isolating metals from the mineral ores in which they were found. There was a need not only to isolate metals for use in tools, weapons and art but also to determine the authenticity and purity of those metals. Along with this came the isolation and utilization of chemical substances, even if their exact nature was not known. Sulfuric acid, known as oil of vitriol, was discovered by the Arabic scientist Jabir ibn Hayyan aroung the 8th century AD upon heating of vitriols - glass like mineral sulfate salts. Phosphorus was isolated from pig urine by the German alchemist Henning Brand in 1669.
In more recent times, there has been an explosion of synthetic methods to pigments and dyes that has paralleled the development of chemistry as a science and discipline. In particular, the discovery of synthetic polymer fibers has created a need for new types of dyes, most of which are organic pigments, that will adhere to those materials. Here is a short synopsis of some of the most important synthetic inorganic pigments. Some of these are also found in nature as minerals, but the synthetic version was often cheaper and more plentiful as well as potentially being free from impurities found in the native minerals.
Cuprorivaite, CaCuSi4O10
Egyptian blue —The Egyptians were quite adept at using natural mineral pigments as these are found in ubiquitously in the artefacts that arose from their civilization, but they were also good at creating new technology. Egyptian blue was a pigment that they prepared by melting silica (SiO2), lime (CaO), alkali (NaOH) and copper. The copper is responsible for its characteristic blue color.
Naples yellow and Chrome Yellow — The chemical formula of Naples yellow is Pb2Sb2O7. While it occurs in nature as the mineral bindheimite, it was rarely used in mineral form. The ancient Egyptians were known to have prepared the pigment, which was widely used from about 1700 to 1850. Its usage fell off with the availabilty of the other synthetic pigments such as lead chromate (PbCrO4, chrome yellow), cadmium sulfide and cobalt yellow. Lead chromate was in use by the early 1800s but was also replaced by the cadmium yellow pigments in use.
Bindheimite, lead antimonate, Pb2Sb2O7
lead chromate, PbCrO4
Potassium cobaltinitrite, K3[Co(NO2)6]
Cobalt yellow — Cobalt yellow, or aureolin, is potassium cobaltinitrite, K3[Co(NO2)6], first prepared by Nicholas Wolfgang Fisher in 1848. It is prepared by oxidation a solution of cobalt(II) salts mixed with potassium nitrite.
CdS1-xSex
Cadmium Yellow Red & Orange — Cd was independently discovered by Friedrich Strohmeyer and Karl Samuel Leberecht Hermann in 1817, but the cadmium compounds did not become widely available until 1840. Cadmium reacts with sulfur to form a simple binary compound CdS, which is bright yellow. This is analogous to mercuric sulfide, which is the pigment vermilion, as cadmium and mercury are in the same group in the periodic table. The most stable for of CdS adopts the same cubic structure as zinc sulfide, ZnS, whose mineral form is called zinc blende. ZnS also forms a hexagonal crystal form that is the mineral wurtzite. It is slightly less stable than the zinc blende form. CdS can also be crystallized in the wurtzite form. Mercuric sulfide on the other hand exists in a trigonal crystal lattice.
By replacing some of the sulfur in CdS with selenium, the color changes from yellow to orange to red with increasing Se content. This is not simply a mixture of CdS and CdSe. The Se atoms replace some of the sulfur atoms in the structure and are statistically distributed throughout the crystal. This is known in solid state chemistry as a solid solution, and the formula is written as CdS1-xSex. Usually these numbers are written such that x denotes a small amount of the dopant element. This substitution process is used to tune the electronic properties of the material, which are also related to the colors observed. Cadmium sulfide is a semiconductor with a band gap of 2.38eV, corresponding to a λ of 520nm. In comparison, cadmium selenide's band gap is 1.74ev and λ = 710nm.
Verdigris — Verdigris includes a number of basic copper acetate compounds that have been manufactured as pigments since the middle ages. The reaction to produce them is quite simple - copper metal is simply placed in vinegar, whose active ingredient is acetic acid, and allowed to stand. Oxidation of Cu0 yields Cu2+ while the H+ ions available from the acetic acid are reduced to hydrogen gas, H2.
CH3CO2H(aq) ⇌ CH3CO2-(aq) + H+(aq)
Cu(s) + 2 H+(aq) → Cu2+(aq) + H2(g)
net reaction: Cu(s) + 2CH3CO2H(aq) → Cu(CH3CO2)2(aq) + H2(g)
Cu3(CH3CO2)2(OH)4
The conditions employed will lead to different compounds which, at first glance, appear to be a simple combination of copper(II) acetate and copper(II) hydroxide as seen in the formulas below. While this is a common way to write these formulas, they are misleading in that the structures are not made up of isolated copper acetate and copper hydroxide units. They are much more complicated as seen in the interactive drawing for one of the compounds shown later on this page. That compound shows complex two-dimensional sheets of copper ions linked by both hydroxide and acetate ions. The colors for each of these compounds is different, ranging from blue to green.
- [Cu(acetate)2]2Cu(OH)2·5H2O, blue
- [Cu(acetate)2]Cu(OH)2·5H2O, blue
- [Cu(acetate)2][Cu(OH)2]2, blue (structure shown below)
- [Cu(acetate)2][Cu(OH)2]3, green
Massicot (or Masticot), Litharge and Minium — Lead oxides have also been used as pigments. Massicot is lead(II) oxide,PbO, while minium is Pb3O4, which has lead in both the +2 and +4 oxidation states. It coud be written as (Pb2+)2Pb4+O4 to emphasize the oxidation states of the lead. Minium is also known as red lead. Massicot was prepared by the Romans by heating lead carbonate. Most metal carbonates undergo this process that is based on the elimination of carbon dioxide gas. It is the same process that is used to prepare lime (CaO) from calcite (CaCO3). Once the PbO is obtained, it can be heated in air to oxidize it to minium. Lead(II) oxide itself exists in two crystalline forms - orthorhombic and tetragonal. The orthorhombic forms produces a yellow powder that is the pigment massicot, while the tetragonal form is known as litharge and is red.
PbCO3(s) → PbO(s) + CO2(g)
6 PbO(s) + O2(g) → 2 Pb3O4(s)
Red lead, Pb3O4
Lead(II) oxide, PbO
PbO
Ferric ferrocyanide, Fe4[Fe(CN)6)]3
Prussian Blue — Ferric ferrocyanide is a dark blue pigment discovered in the early 1700s. It was produced originally via the formation of iron(II) ferrocyanide, Fe2[Fe(CN)6], an insoluble white compound used as a pigment known as Berlin white. The subsequent oxidation of this compound produces ferric ferrocyanide, Fe3+4[Fe2+(CN)6]3. Today it can be made more simply in the lab by reacting aqueous iron(III) chloride, FeCl3, with K4[Fe(CN)6]. It gets its color from charge transfer that takes place upon absorbtion of red-orange light. In this process, the absorption of light causes an electron to move from the Fe2+ in the [Fe(CN)6]4- to the Fe3+ counterions. The compound is highly insoluble in water and forms a precipitate. As as pigment, it was originally known as Berlin blue, but became known as Paris or Parisian blue when used in oil paintings. Turnbull's blue is the same compound but it is prepared by adding Fe2+ salts to salts of the ferricyanide ion, [Fe(CN)6]3+.
Cerulean ble was another blue pigment that was discovered in 1789 by Swiss chemist Albrecht Höpfner. It is cobalt stannate, CoSnO3 and became popular for painting skies.

Cobalt stannate, CoSnO3
French Ultramarine Blue — In 1826, the French chemist Jean-Baptiste Guimet created a substitute for lapis lazuli, known up until that time as ultramarine. He heated kaolinate, with sodium carbonate and sulfur. Kaolinite, Al2(OH)4Si2O5, provides the aluminosilicate framework while the sodium carbonate provides the alkali metal cations and additional oxygen. The sulfur provides the source for the S3- radicals that are the source of the blue coloration. The result was a vibrant blue pigment that was in many regards superior to the natural mineral, even though the synthetic version is based on the same chemistry. To distinguish the synthetic material from the mineral-based pigment, the new compound was marketed as French ultramarine blue in homage to the place of its origin.
Phthalo Blue and Phthalo Green — In the 20th century chemists discovered the active light-harvesting center in green plants. It is shown in the figure to the right. Four nitrogen atoms point towards the center of the molecule, and the size of the space between the nitrogen atoms is ideally suited for bonding to a metal cation, which is Mg2+ in the case of the chlorophylls. This unit is common to all of the chlorophylls, although there are side chains attached to the ring that have different organic groups that result in slightly different green colors. Leaves turn colors in autumn because the chlorophyll molecules decompose leaving other natural pigments to be observed.
Another term used to describe the organic ligand in chlorophyll is chlorin, and it belongs to a general classification known as macrocyles, ring-like organic molecules that can bond to metal ions. Over the past several decades, there has been a considerable amount of research done in studying macrocyclic ligands with a large range of different transition metals and p-block metals bound to them.
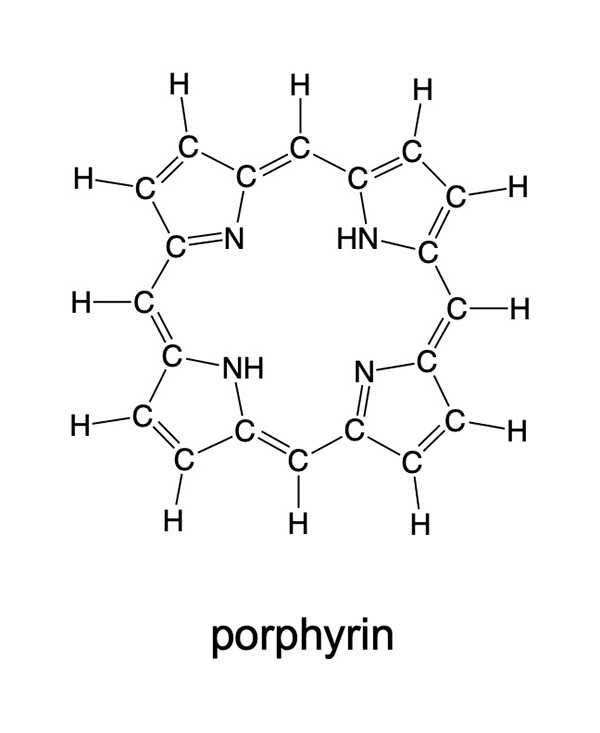
Other macrocycles related to chlorin that are used for pigments and dyes include porphyrin and phthalocyanine, and their derivatives. While transition metals bound in the center of the ring affect the color, the network of multiple bonds in the molecule create color as well. The metal replaces the two hydrogen atoms attached to the nitrogens pointing towards the center of the ring, so one can think of the porphyrin as the negatively charged ion [C20N4H12]2- when it binds a metal ion. Porphyrins related to the macrocycles present in chlorophyll are present in many living organisms. For example an iron-porphyrin complex is the active center in the hemoglobin molecule that transports oxygen in the blood. These molecules often have other organic groups replacing the hydrogen atoms on the periphery of the molecule that allow them to bind to other biological molecules.
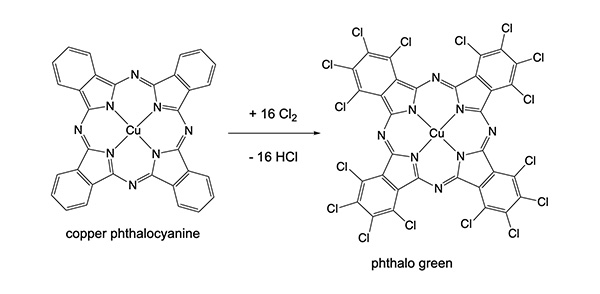
A closely-related molecule is phthalocyanine. It has additional nitrogen atoms in the ring system. Its metal compounds have become common inorganic pigments, including phthalo blue and phthalo green. The syntheses of these two compounds are given below. Phthalo blue is readily converted into phthalo green by reaction with chlorine, although complete chlorination of the ring is hard to achieve and they are not readily separated because of their insolubility. The actual phthalo green pigment will be a mixture of varying degrees of substitution – Cu[C32N8Cl16-xHx] – and consequently display different shades of green.
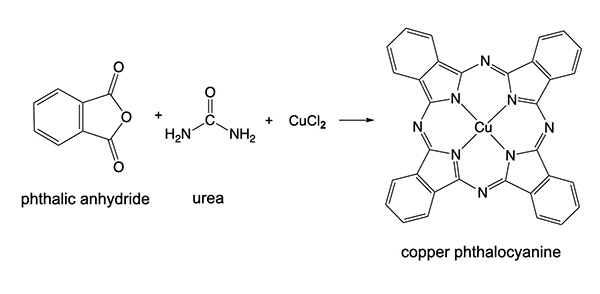
Copper phthalocyanine, CuC32N8H16
Copper hexadecachlorophthalocyanine, CuC32N8Cl16
Chromium(III) oxide, Cr2O3
Chromium and Cobalt green — Cobalt green was first prepared in 1780 by the Swedish chemist Sven Rinman. It is prepared by doping some cobalt(II) oxide into zinc oxide, ZnO. The formula is Zn1-xCoxO and for less than x = 0.3, the compound is bright green. It is, however, more expensive and not as highly colored as chromium green. Chromium green is chromium(III) oxide, Cr2O3 that was first used in the mid 1800s as a pigment. It is known sometimes as veridian.

Scheele's Green — Scheele's green, also known as Paris green, is a bright pigment synthesized by the German pharmacist Carl Wilhelm Scheele in 1775. It was not only used as a pigment in paints but also for fabrics, producing a stunning green color that was in high demand in the middle 1800s. Its nominal composition is CuHAsO3 but the crystal structure has not been determined, and it appears to be a mixture of a number of different compound with varying Cu-As ratios. The oxidation number on arsenic is +3 in this compound and it is prepared from treating a solution of potassium carbonate and As2O3 with copper sulfate. Because of the presence of arsenic, it is a toxic compound and has been implicated in the death of Napoleon. The rooms of his house on St. Helena where he was exiled were covered with wallpaper containing the pigment. It was later shown that microorganisms could convert the pigment into gaseous, highy toxic arsine (AsH3) and methylated arsine compounds - As(CH3)3-x (x = 1-3). Hair samples from Napoleon later showed high levels of arsenic, and arsenic poisoning is linked to higher instances of stomach cancer, which may have been his actual cause of death. This pigment has largely been replaced by less toxic and more stable green pigments. Attempts were made to make an improved Scheele's green and the pigment emerald green was produced in 1808. It is a copper(II) acetoarsenite and was prepared by treating verdigris with arsenic compounds.
Copper acetoarsenite, Cu2(CH3CO2)2(As3O6)2
The unique atoms of emerald green showing the As3O62- ring unit
Identification of Inorganic Pigments
Identification of inorganic pigments can be done in several ways and often more than one technique is required to fully identify a particular compound. X-ray fluoresence spectroscopy is useful for identifying the metallic elements involved in particular pigments. Heavy metals such as mercury and lead are particularly easy to identify with XRF. Often the elemental composition coupled with the color of the pigment will lead to a good first guess of what the pigment is. But some elements will be found in a number of different pigments, such as copper which is responsible for the green or blue color in many materials. If one can get a small sample of the pigment from a painting, then optical or electron microscopy can be useful for exmamining the shapes of the crystallites in the pigment, which are characteristic of a particular crystalline form. If a larger sample is available, such as a powdered sample of pigment from an artist's studio, X-ray powder diffraction can identify the crystalline materials present. A more in-depth discussion of these techniques is available on the page Analytical Methods.
Shortcuts
Resources and References
- Webexhibits: Pigments through the Ages.
- ColourLex: Paintings. Pigments. Methods.
- "Arsenic and Old Tastes Made Victorian Wallpaper Deadly - Victorians were obsessed with vividly-colored wallpaper, which is on-trend for this year–though arsenic poisoning is never in style", Eschner Kat, Smithsonian Magazine, April 3, 2017.
Crystal Structure Data
- Structures for which crystallographic information format (CIF) were available were obtained from the Inorganic Crystal Structure Database (ICSD) or the American Mineralogist Crystal Structure Databaseand the figures and .pdb files were created using CrystalMaker.
- Aureolin, cobalt yellow: Pertlik F, Zeitschrift fur Kristallographie, Krystallphysik, Kristallchemie 1977 145, 35.
- Azurite: Belokoneva E L, Gubina Y K, Forsyth J B, Physics and Chemistry of Minerals 2001 28, 498 - 507.
- Baryte: Colville A A, Staudhammer K, American Mineralogist 1967, 52, 1877 - 1880.
- Bindheimite, Lead antimonate: Kuznetsov, V G, Koz'min, P A, Zhurnal Neorganicheskoi Khimii, 1958, 3, 2361 - 2365.
- Celadonite and glauconiate: Drits V A, Zviagina B B, McCarty D K, Salyn A L American Mineralogist 2010 95, 348 - 361.
- Chlorin: Chow, H-C, Serlin R, Strouse C E Journal of the American Chemical Society 1975, 97, 7230 - 7237.
- Cinnabar: Auvray P, Genet F Bulletin de la Societe Francaise de Mineralogie et de Cristallographie 1973, 96, 218 - 219.
- Cobalt stannate: Jain A, Ong S P, Hautier G, Chen W, Richards W D, Dacek, S, Cholia S, Gunter D, Skinner D, Ceder G, Persson K, APL Materials, The Materials Project: A materials genome approach to accelerating materials innovation 2013 011002, DOI: 10.1063/1.4812323
- Chromium green, Cr2O3: Sawada H, Materials Research Bulletin 1994, 29, 239 - 245.
- Crocoite, PbCrO4 Quareni S, De Pieri R Acta Crystallographica 1965, 19, 287 - 289.
- Copper phthalocyanine: Brown C J, Journal of the Chemical Society A 1968, 2488, DOI: 10.1039/j19680002488
- Cuprorivaite: Chakoumakos B C, Fernandez-Baca J A, Boatner L A, Journal of Solid State Chemistry, 1993, 103, 105 - 113.
- Gypsum: Schofield P F, Knight K S, Stretton I C American Mineralogist 1996 81, 847 - 851.
- Hawleyite, CdS: Skinner B J, American Mineralogist, 1961, 46, 1399 - 1411.
- Hematite, Fe2O3: Maslen E N, Streltsov V A, Streltsova N R, Ishizawa N, Acta Crystallographica, B 1994, 50, 435 - 441.
- Kaolinite Gruner W (1932) _cod_database_code 1011045. Zeitschrift fur Kristallographie 1932, 83, 75 - 88.
- K3[Co(NO2]6]: van Driel, M; Verweel, H J, Zeitschrift fuer Kristallographie, 1936, 95, 308 - 314.
- Lazurite: Tarling S E, Barnes P, Klinowski J, Acta Crystallographica B, 1988, 44, 128 - 135.
- Lead white, PbCO3: Canadian Mineralogist, 2009, 47, 1245 - 1255.
- Litharge, PbODickinson R, Friauf J, Journal of the American Chemical Society, 1924, 46, 2457 - 2462.
- Malachite: Zigan F, Joswig W, Schuster H U, Mason S A Zeitschrift fur Kristallographie, 1977, 145, 412 - 426.
- Massicot, PbO: Hill R J, Acta Crystallographica, C, 1985, 41, 1281 - 1284.
- Minium: Dinnebier R E, Carlson S, Hanfland M, Jansen M, American Mineralogist, 2003, 88, 996 - 1002.
- Naples yellow: Schenk H, Dik J, Peschar R, Acta Cryst. 2005, A61, C494.
- Orpiment: Mullen D J E, Nowacki W, Zeitschrift fur Kristallographie, 1972, 136, 48 - 65.
- Phthalo green: Löbbert G, "Phthalocyanines", Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. (2000). doi:10.1002/14356007.a20_213
- Prussian blue: Weiser H B, Milligan W O, Bates J B Journal of Physical Chemistry, 1942, 46, 99 - 111.
- Realgar: Kyono A, Kimata M, Hatta T, American Mineralogist, 2005, 90, 1563 - 1570.
- Titanium dioxide - Anatase: Howard C J, Sabine T M, Dickson F, Acta Crystallographica B, 1991, 47, 462 - 468.
- Titanium dioxide - Rutile: Meagher E P, Lager G A, The Canadian Mineralogist, 1979, 17, 77 - 85.
- Verdigris: Bette S, Kremer R K, Eggert G, Tang C C, Dinnebier R E, Dalton Transactions, 2017, 46, 14847 - 14858.
- Zinc white, ZnO: Kihara K, Donnay G, The Canadian Mineralogist, 1985, 23, 647 - 654.
Credits for the Header Image
- Azurite on Malachite, partial image from Géry Parent, Public Domain, Wikipedia.
- Cinnabar, partial image from Rob Lavinsky, iRocks.com, CC-BY-SA-3.0/CC BY-SA.
- Realgar, partial image from Géry Parent CC BY-SA (https://creativecommons.org/licenses/by-sa/4.0)
Department of Chemistry

Houston, TX
6100 Main St., Houston, TX 77005-1827 | Mailing Address: P.O. Box 1892, Houston, TX 77251-1892 713-348-0000 |












