
In chemistry, the term glass can refer to any amorphous material that does not exhibit long-range order in the solid phase. But glass in common terms usually refers to amorphous silicon dioxide, SiO2. Silicon dioxide in mineral form is quartz or, less frequently, cristobalite (See Silica-based Gemstones. These are a crystalline materials with a definite crystal lattice. Erosion of the minerals leads to tiny particles of the minerals forming sand, and the first glasses were made by melting sand at high temperature. Glasses are not always transparent. The silica after it has been heated to melting can be formed into various shapes. These glasses must be cooled slowly so that cracking does not occur. The strain present in glass objects can be reduced by holding the object near but not above the melting point of the glass in a process called annealing.
As with the crystalline forms of silica, the silicon atoms in the glass are tetrahedral and each is bound to four oxygen atoms, each of which bridges to another silicon atom. In the glass form of SiO2, there is no long range order in the arrangment of the network of Si and O bonds. Other oxides of boron, aluminum, lead, sodium, potassium, cobalt and iron may be included with silica in forming a glass. The transition metals will produce colored glasses. The properties of the glasses are dramatically altered by the combination of elements present. Laboratory glassware and kitchen cookware are made of heat resistant borosilicate glass. Pure silica, which is known as quartz glass, has a very high melting point. The (see Table of Glass Compositions and Properties) compares properties for glasses of various compositions. Lead glass crystal was quite popular form making cut glass goblets, decanters, bowls and vases because of its clarity, but lead can leach from glass slowly, so liquids - especially those containing alcohol - should not be stored in lead glass crystal for any length of time before serving them.
Coloring glass was developed to an high art during the Middle Ages for its used in stained glass windows for cathedrals and other important buildings. Often the colors are due to the presence of different metal ions and that color was highly dependent on the oxidation state of the ions. The presence of iron will produce green glass if both Fe2+ and Fe3+ are present, but having all Fe3+ will result in yellow to deep brown glass such as that used in drink bottles. Ancient Roman glass often has a slight green color due to the presence of iron. Deep blue glass results when cobalt is added. Manganese can produce yellow (Mn2+) or purple colors (Mn3+), while a mixture of the two oxidation states results in pink glass. Copper in its low oxidation state would produce red glass, while Cu2+ gives a deep blue glass. The relative amounts of the metal oxidation states would be controlled by controlling the environment in which the glass was prepared. An atmosphere rich in oxygen would produce the higher oxidation states, while reducing environments would favor the lower oxidation states. Recently, scientists have also discovered that some stained glass colors from the Middle Ages were due to gold or silver nanoparticles (small aggregates of metal atoms less than ~100nm, or 100 x 10-9m). The nanoparticles were obtained by adding gold chloride or silver nitrate to the glass. Because these are both noble metals and hard to oxidize, upon melting the glass would, the metals are reduced and the resulting metal atoms aggregate to give nano-sized particles. Gold produces a brilliant red color and silver gives bright yellow.

_479px.jpg)

There are two important ways in which glass objects are crafted. One is cutting, which involves creating intricate patterns that reflect light. in modern times, the cutting process involves holding the glass against a moving wheel that is embedded with an abrasive such as diamond grit. This has been most notably done with leaded glass to achieve spectacular effects. Etching can also be used to decorate glass objects. In this process, a design is first created on the glass using a mask, such as a wax. The mask is placed on areas of the glass that are not to be etched in the process. The remaining exposed glass is then treated with hydrofluoric acid, often made in situ by mixing ground fluorite (CaF2) with a strong acid such as sulfuric acid (H2SO4). Hydrofluoric acid reacts with the SiO2 in the glass creating soluble [SiF6]2- salts, which are then washed away. The remaining glass takes on a frosted appearance.

_MET_DP108438.jpg)
Ceramics include pottery and porcelain made from clays, which are a type of aluminosilicate mineral. While clays are easy to shape into a variety of forms when wet, after drying and heating in a kiln, they become quite hard and water tight. The hardening process involves the removal of water between the aluminosilicate structures that links them into a covalent network of aluminum, silicon and oxygen ions. The resulting network is much harder than the starting material. Transition metal ions such as iron can also be found in these structures, and this will give the clay a red or brown color. One of the challenges in making fine porcelain was to create a clay ceramic that was free of iron so that the color would be pure white.
Clay-based ceramics fall into three general classes: earthenware, stoneware and porcelain. Earthenware is a clay object that has been heated in a kiln in a process known as 'firing' that is done at a relatively low temperature (up to about 1200°C). Earthenware will still absorb water. A common example is a clay pot for planting flowers. Stoneware results from firing at a slightly higher temperature, up to ca. 1300°C, while fine porcelains are generally fired at even higher temperatures, 1200-1400°C. The actual temperature ranges used will depend on the clay that is used. The production of fine porcelain that is white and translucent was first prepared by the Chinese. This type of product uses kaolin clay that is low in the metal ions that give common clays their color. The definition of porcelain that is used in describing Chinese pottery is not uniform so that the exact date of the first official Chinese porcelain is up for some debate. The development of porcelain appears to have begun as early as ca. 200 B.C. and by about 200 A.D., the Chinese had developed kilns that would achieve temperatures up to 1300°C. Chinese porcelain making was unrivaled for centuries and large quantities were exported to Europe. Because of the cost and size of the European market, efforts began in Europe to break the Chinese porcelain monopoly. In the early 1700s, the first European hard-paste porcelain was developed in Meissen. This was followed by other countries where manufacturers such as the French Manfacture de Vicennes. This company was relocated in the 1750s to Sèvres, and the new styles it produced had become very popular by the 1760s under the guidance of Madame du Pompadour.
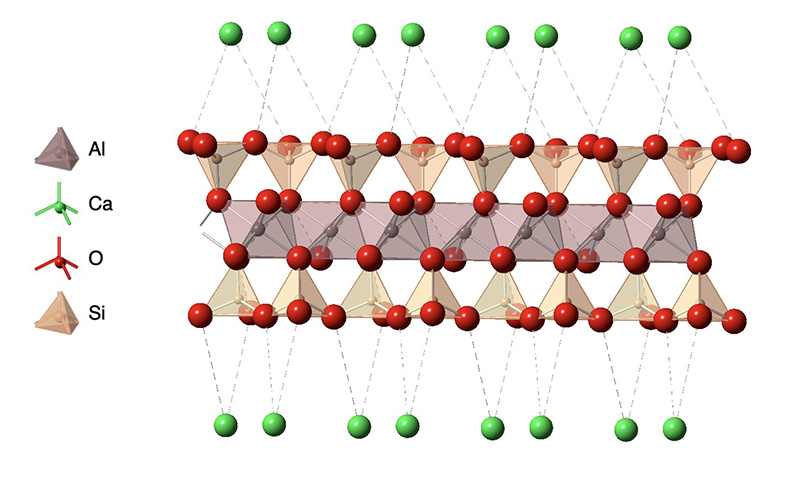
One example of a clay is montmorillonite whose structure is shown in the figure. As with many clay minerals, the structure is composed of anionic aluminosilicate layers held together by metal cations such a calcium, sodium, potassium and magnesium in their common oxidation states (Ca2+, Na+, K+, Mg2+). The type of representation show in the figure is known as a polyhedral representation in which the faces of the polyhedron between atoms bonded to a metal ion are illustrated in color. This figure shows the coordination polyhedra around the silicon and aluminum atoms in the aluminosilicate layeras. Each silicon is bonded to four oxygen atoms that form a tetrahedron with the Si in the center, while the aluminum is bound to six oxygen atoms in an octahedral arrangement.

During the firing process the clay is altered chemically. Clay begins as a hydrated aluminosilicate that is made of many tiny particles that have -O-H groups covalently attached to their surfaces. When these tiny particles are heated, cross-linking of the particles occurs with accompanying loss of water via the combination of two -O-H groups: X-O-H + H-O-Y → X-O-Y + H-O-H, where X and Y or either a Si or Al. This results in covalent oxygen bridges between the particles that stabilize the overall structure, as illustrated schematically in the figure.
Glazes are chemical compounds that react with the surface of a ceramic during to form a glass. They may either react with the surface of the ceramic to form a glass, or they simply be a glass-forming substance that melts onto the surface upon heating. These glasses may be colored, usually by the presence of metal ions in the glass. Some common examples are iron oxides which produce a brown glaze, cobalt whose glazes are blue, and manganese that gives a purple color. A simple method of glazing is a salt glaze, which has been known since the Middle Ages. In this method, sodium chloride is placed in the kiln when an object is fired. The sodium chloride volatilizes and the Na+ ions react with the surface of the clay, resulting in the formation of a glassy sodium silicate layer on the surface of the object.

There are several approaches to glazing a ceramic object. The clay object before it is fired is known as greenware. The greenware may be subjected to an initial firing, and the resulting object is known as biscuit-ware. An underglaze may be applied to the either the unfired greenware or the biscuit-ware. Often the underglaze is in a form known as slip, which is a mixture of clay and water that has a consistency suitable for painting the slip on the ceramic ware. When fired, the glaze melts and fuses with the surface of the ceramic. Additional glazes may be painted on top of the underglaze and re-fired, forming a smooth surface.

Maria Montoya Martinez of the San Ildefonso Pueblo in New Mexico created techniques for making black pottery beginning in the 1920s. Her work developed over decades and was a complex process both in creating the greenware object as well as in the firing. The firing process involves a reducing atmosphere which allows incompletely combusted carbon compounds to with metals in the clay or in the slip coating the object. This reduces the metals generating the black color. The reducing atmosphere in the Martinez's work was created by adding organic matter to the kiln during the firing process. In commercial metal refining, a reducing atmosphere is achieved by reducing the amount of oxygen entering the kiln as well as providing reducing gases such as CO and H2. The black pottery that Martinez created became highly valued for its artistry and became valuable collector's items.

Greek black-painted pottery used a similar technique employing three stages for firing for the creation of intricate images. The object was prepared and painted with a slip that would ultimately fire to give the desired black color. The first stage of firing would be oxygen rich and resulted in an object that was red-orange overall, including the fired slip. The next stage involved raising the temperature and adding green wood to the kiln to create a reducing atmosphere. This turns the object black, both the body of the object and the painted image. After this state, the vents on the kiln were opened to allow more oxygen to enter and the temperature was reduced. While the body of the object returned to its red-orange color from the oxidizing environment, the painted slip remained black because it had already vitrefied and was no longer subject to further oxidation. The black could be painted the object as either a positive or negative image, and slips that turned other shades of red or white were also included in the painting stage.
Shortcuts
Resources and References
- https://Webelements.com
- The Elements, John Emsley, Oxford University Press, 3rd Edition, 1998, ISBN-13: 978-0198558187, ISBN-10: 019855818X.
- Colored Gold, Wikipedia. sso
- American Cut Glass Association
Crystal Structure Data
- Cementite, Fe3C, Fruchart D, Chaudouet P, Fruchart R, Rouault A, Senateur J. P. Journal of Solid State Chemistry, 1984, 51, 246 - 252.
- Fe4C0.63, Nagakura S, Toyoshima M, Transactions of the Japan Institute of Metals, 1979, 20, 100 - 110.
Credits for the Header Image
- Black-Figure Amphora Depicting Theseus Slaying the Minotaur and the Gods Departing for Olympu, Antimenes Painter, Greek, 5310-510 BC, From the Collection of the Museum of Fine Arts Houston, Public Domain.
- Vase, Louis Comfort Tiffany, c. 1892-1893, From the Collection of the Museum of Fine Arts Houston, Public Domain.
- Amhporiskos, Unknown, Roman, 300-500 AD, From the Collection of the Museum of Fine Arts Houston, Public Domain.
- Figure of a Boy Farmer, Meissen Porcelain Factory, Hard-paste porcelain, From the Collection of the Museum of Fine Arts Houston, Public Domain.
- Figure of a Horse, Unknown, Chinese, early 7th to early 10th century, Earthenware with three-color (sancai) glaze, From the Collection of the Museum of Fine Arts Houston, Public Domain.













