Fibers
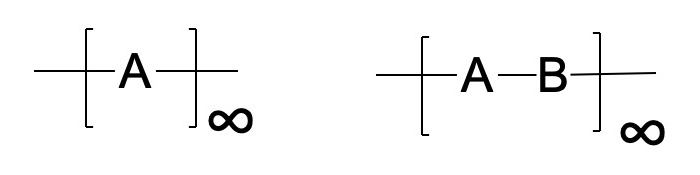
All textiles and paper are based on molecules that can be combined to form fibers. Whether naturally-occurring or man-made, these fibers are made up of polymers. Polymers are long strings of small organic molecules, known as monomers, that are connected end-to-end to produce very long covalently-bound strands. There is a limitless variety in the way polymers are put together. A polymer can be made up of a single monomer (here labelled A or B), or the polymer can be made up of more than one type of monomer. If more than one type of monomer is present, the polymer can have a regular repeating of the monomers such as -A-B-A-B-A-B- etc., or they can be connected randomly: -A-B-B-B-A-A-B-A-A-, etc. Different monomers can be polymerized in segments to give block copolymers, -[A]x-[B]y-[C]z-, etc. Proteins are polymers composed of the 20 different naturally-occurring amino acids, and the amino-acid sequence is critical to determining the function of the molecule. Polymers may form in straight chains, but in some cases they can also be branched, meaning that they have side polymers that fork off at various points along the main chain.
Natural fibers
Natural fibers have been harvested, processed and turned into textiles for thousands of years. The first linen fabrics have been dated to the Bronze Age. Natural fibers fall into two main categories: cellulose- and protein-based. The cellulose-based materials derive from, plants and include cotton and linen, derived from flax, while wool and silk are protein-based animal products.

Cellulose is a polymer of glucose that forms fibers providing structural stiffness for plants and trees. Cellulose is related to starch, which is also a polymer of glucose, but the glucose molecules in the chains are slightly different. In starch, they connect via α linkages while those in cellulose are the β form. If one examines the α and β forms of glucose in the figures below, one will see that the -OH group on the carbon adjacent to the oxygen atom in the ring and the -CH2OH groups are on opposite sides of the ring in α-D-glucose but are on the same side of the ring in β-D-glucose. While this seems like a very minor change in structure, it has a major impact on the properties of the two molecules. Starch is water soluble but cellulose is not, consequently starch can be digested but not cellulose. Additionally, the glucose polymer in starch may have branches created by attaching additional glucose molecules along the sides of the chain. The straight chain form of starch is called amylose and the branched form is amylose pectin. Another commonly encountered polymer of glucose is glycogen. Glycogen contains branched polymers of glucose attached to a central protein core called glycogenin. Putting glucose into glycogen is how the body stores glucose in a compact form.

In addition to cellulose, the natural plant fibers in cotton and linen also contain hemicellulose, lignin, pectin and ash (see Wood for a description of their structures). The processing of these fibers removes these other materials by mechanical and/or chemical methods. A major difference between cotton and linen is the length of the fibers. Cotton fibers are shorter, coming from the boll of the cotton flower, while linen fibers are considerably longer as they are produced in the stems of the plants. Consequently, cotton is less durable than linen, but linen is less elastic. Dried flax plants are first threshed to remove the seeds, and then they are retted to free the fibers from the stalk, after which the fibers are separated from the straw. The flax plant has an additional importance to Art as flax seeds are the source of linseed oil used in paintings.

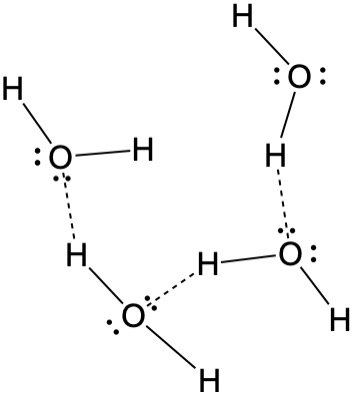
One of the important features of the cellulose polymer is the presence of numerous -O-H groups along the chain. This gives rise to the possibility of hydrogen-bonding. Whenever hydrogen atoms are attached to an electronegative atom such as oxygen, nitrogen or fluorine, that hydrogen becomes slightly positively charged. This creates a slight dipole moment that allows that hydrogen to be attracted to electronegative atoms possessing lone pairs of electrons, and these interactions can be both intramolecular and intermolecular. They are stronger than many other types of dipole-dipole forces, and hydrogen bonds become a facile way for hydrogen to be transferred from one molecule to another. In aqueous solution, these hydrogen atoms are constantly exchanging with each other and the the hydrogen atoms of the water. The unique properties of water are due in large part to hydrogen bonding between the water molecules. These hydrogen-bonding interactions hold the cellulose molecules together in a more rigid structure than would be possible otherwise. Hydrogen bonding is also important for proteins where hydrogen bonds involving -O-H···N, -N-H···O and N-H···N interactions in addition to -O-H···O are important in determining and stabilizing the protein structure. The long chains of amino acids fold and coil in such a way as to give a very well-defined structure that is crucial to the function of the protein, and this structure is held in place largely by hydrogen bonding. When this bonding network is disrupted, the proteins are said to be denatured, which is the process that takes place when eggs are cooked. In that case, the protein albumin is denatured from a clear viscous liquid to produce a white solid.
Polar groups like O-H and N-H are also important for binding dyes to fibers (see Pigments & Dyes.). The lone pairs of electrons on the oxygen and nitrogen atoms may bond to metal cations such as Al3+ and Fe3+ that serve as mordants whose function is to bind to both the lone pairs on the fiber and those on the dye molecule to secure the dye in the textile.
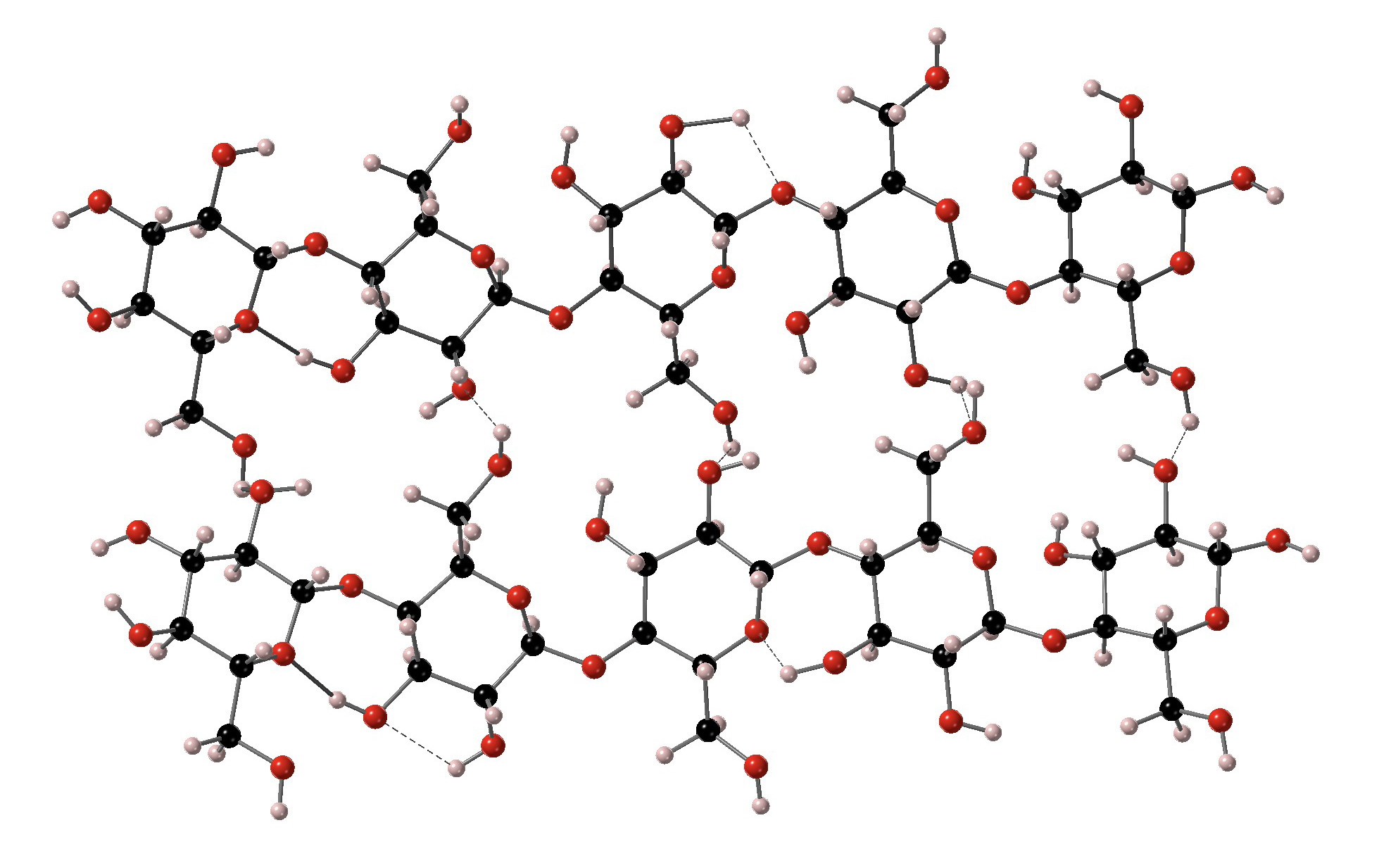
The cellulose in the wood of trees has long been used essentially as it is chemically for the production of furniture. This cellulose is supported by plant cells that have complex structural functions. Other components like lignins, hemicellulose and pectins are important in determing the color and grain patterns in furniture and decorative objects. These structures are described in more detail at Wood.
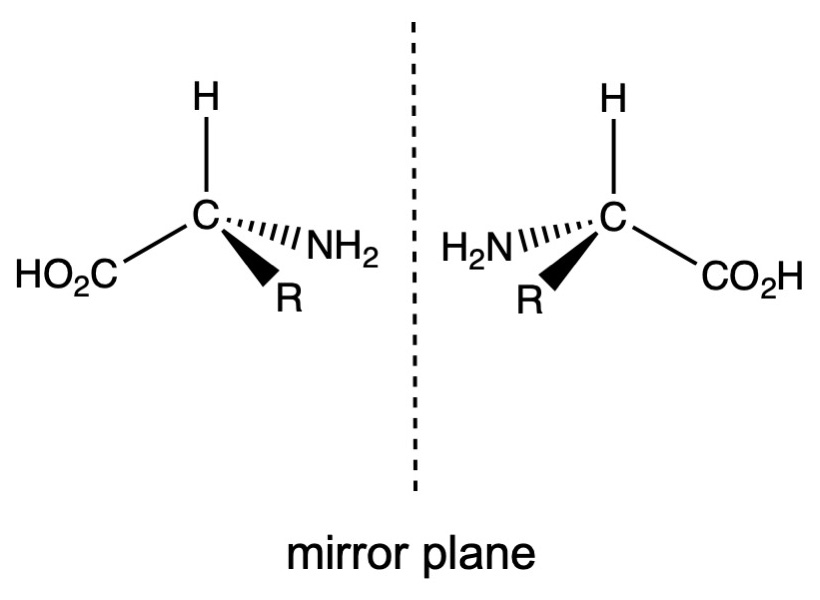
Proteins are polymers made up of strings of amino acids. There are 20 naturally-occurring amino acids, and these have a general form of C(R)(H)(NH2)(CO2H). The NH2 group (shown in blue in the table) is known as an amine (derived from ammonia, NH3) and the -CO2H group (green in the table) is a carboxylic acid function, hence the name amino acid. These molecules, except for glycine are chiral. What that means is that a carbon atom that has four different groups attached to it will have two isomers known as enantiomers, which are non-superimposable mirror images of each other. Glycine is not chiral because it does not have four different groups attached to the central carbon atom, since the R group in this case is H. In nature, only one of these two isomers is normally formed. The two forms are designated as L and D, based on the relative orientations of the four groups. All naturally-occurring amino acids have the L form. The enantiomers will have identical physical properties except for one which is judged by their effect on plane polarized light. A solution of one pure enantiomer will rotate the polarized light in one direction, while the other enantiomer will rotate the light the same amount in the other direction. Those that rotate light towards the left are known as levorotary and those that rotate to the right are dextrorotary. Under normal laboratory conditions, a reaction would produce equal amounts of these two isomers and the mixture would be called racemic and show no optical rotation, because the individual rotations of equal numbers of molecules with different chirality would cancel. Unlike the normal laboratory conditions, Nature has fine-tuned the production of these chiral molecules so that only one is enantiomer is formed.
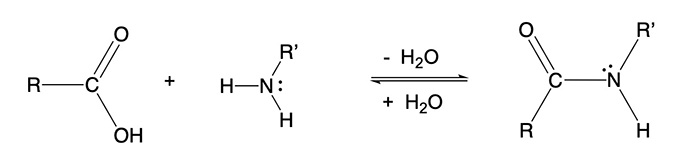
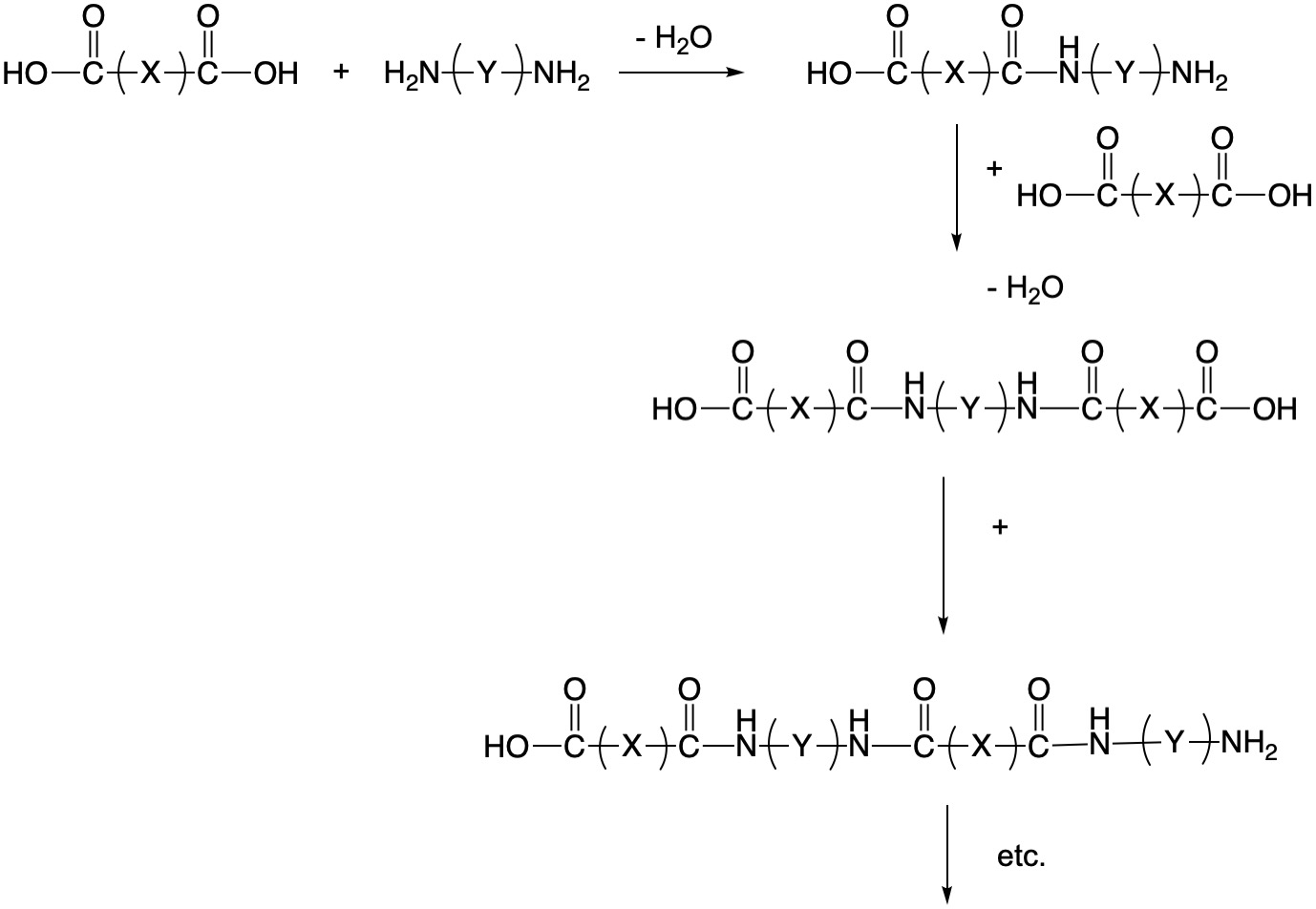
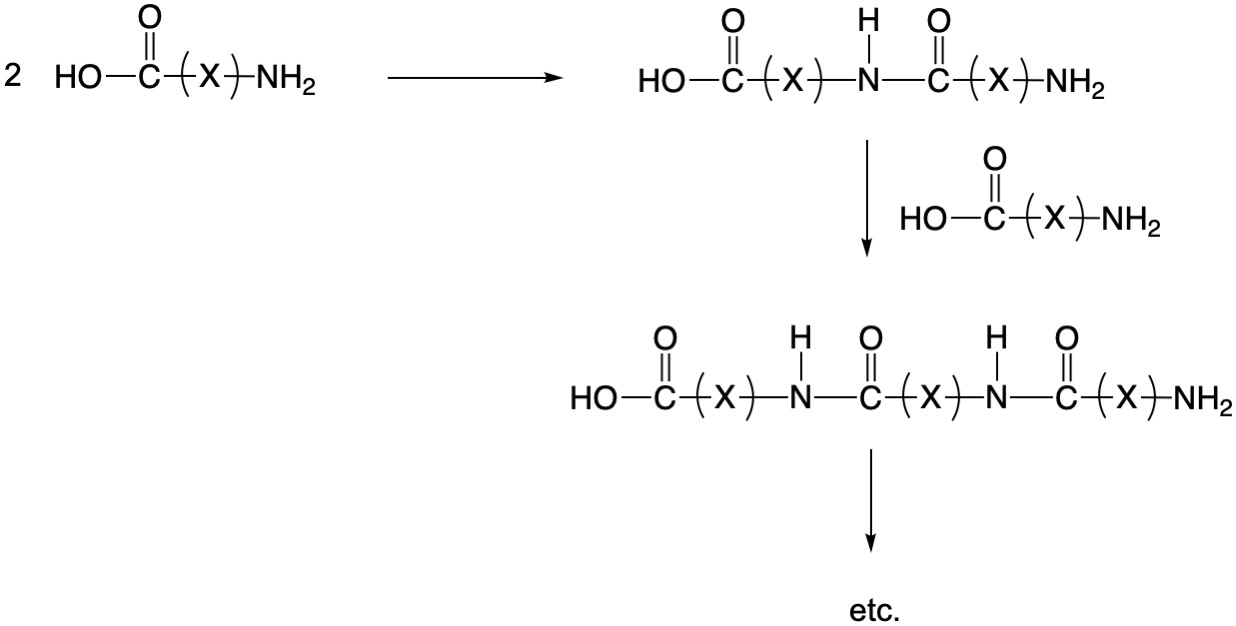
The amino acids are connected by peptide linkages, which is also known as an amide functional group in organic chemistry. These are formed when a carboxlic acid reacts with an amine with the elimination of water. In general processes in organic chemistry that eliminate water are known as condensation reactions. The reaction is reversible as water can re-add to the amide bond, and the result is known as hydrolysis. Some man-made polymers such as the various nylons also make use of the formation of amide bonds. The formation of a polymer occurs because the amino acids have both carboxylic acid and amine groups, so that they can link end to end.
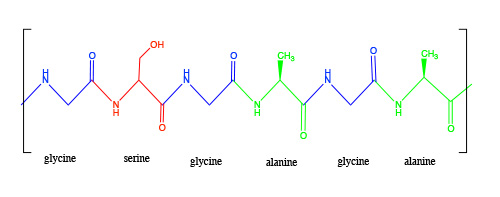
Natural silk fibers are harvested from the coccoons of several varieties of moths, including Bombyx mori, Hyalophora cecropia, Antheraea pernyi and Samia cynthia. The silk fiber is a protein-based polymer called fibroin that is coated with a waxy substance called siricin. Fibroin contains a regularly repeating sequence of glycine, serine and alanine in the order [-glycine-serine-glycine-alanine-glycine-alanine-]∞. The structure naturally coils into what is known as an α-helix, which then further assembles via hydrogen bonding into a more complex structure know as a β pleated-sheet.


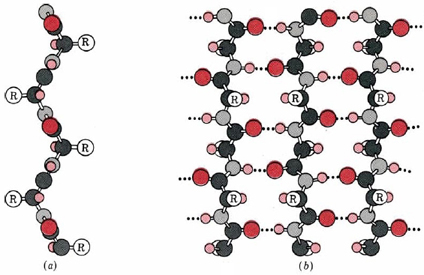
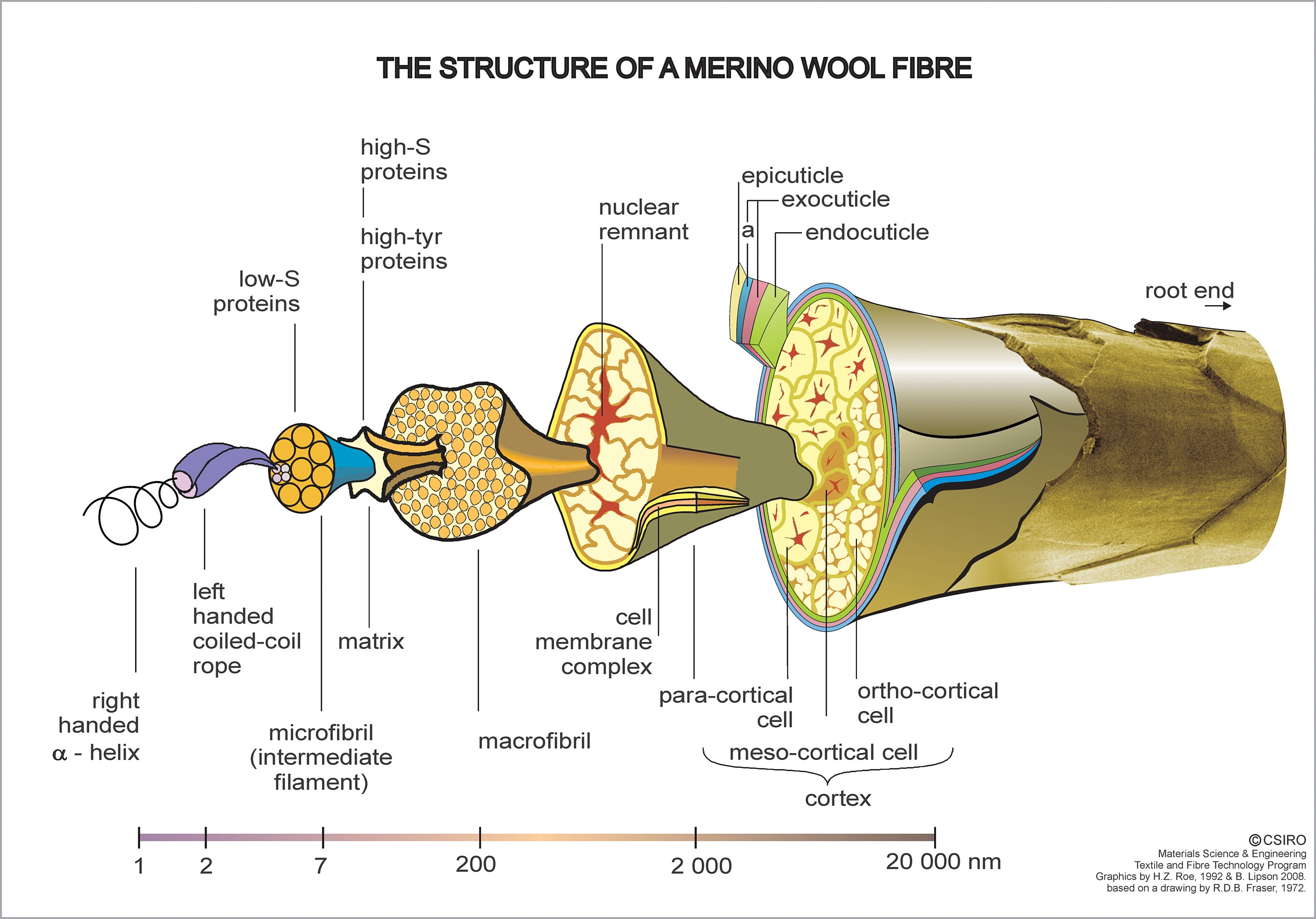
Wool has a complex structure that involves a central protein core surrounded by a series of cellular structures. The protein, like silk, belongs to a class called keratins. These proteins also form α-helices and β pleated sheet structures. Additionally, the proteins in wool contain a high percentage of cysteine (see above), an amino acid containing an S-H side chain, known as a thiol group. These groups are important in the structure of these proteins because the S-H groups from cysteine residues on nearby sections of the polymer can combine to form disulfide linkages, -S-S-. These are covalent bonds that hold the structure together more tightly than hydrogen bonding does. From the figure of wool it can be seen that the protein polymers form coils within coils that are in turn coated with a matrix. These structures are in turn bundled into microfibrils and embedded into a cellular network that help determine the overall macroscopic characeristics of the fiber.
Man-made fibers
One of the first synthetic polymers was derived from cellulose extracted from wood chips. The product is rayon and was invented by Hilaire de Chardonnet, a French scientist, who discovered that cellulose could be made water soluble by treating it with base and carbon disulfide, CS2. This process creates viscose linkages, also known as xanthates – O-CS2-, by attaching the CS2 to the CH2OH groups on the glucose units. Each xanthate group has a negative charge that is balanced by a sodium cation. This means that the cellulose chain overall has multiple negative charges and becomes water soluble as a result. The process, however, is reversible. When a solution of the dissolved viscose is placed in acid, the xanthate groups are eliminated and the cellulose is regenerated. When this is done by passing a solution of viscose through a spinerette, a device very similar to a showerhead, into an acidic solution, fibers are produced that are very similar in feel to silk. Rayon was marketed as a silk substitute that was much less expensive.
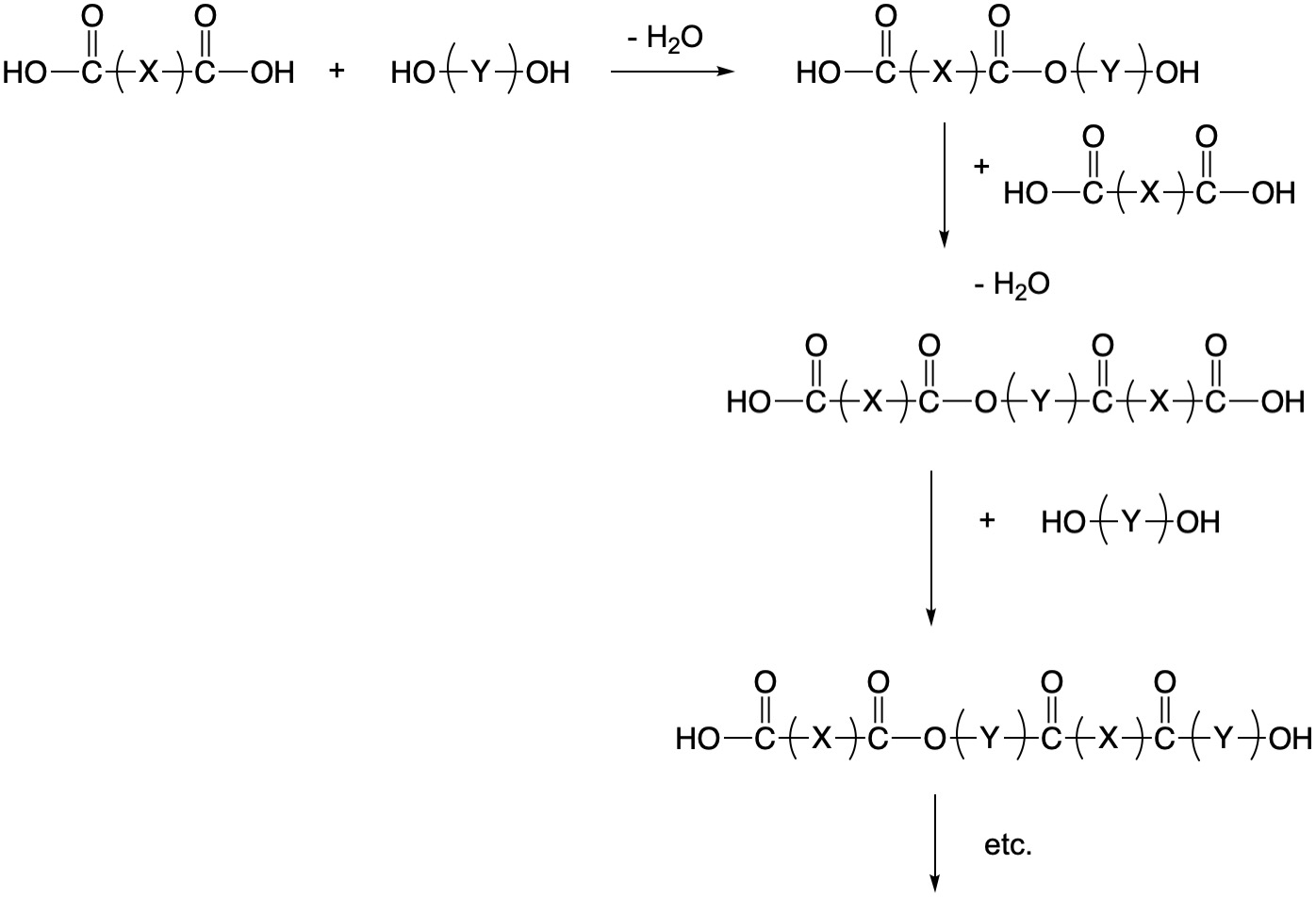
As an understanding of the structure of natural polymers developed, chemists were also developing ever-more sophisticated synthetic methodologies. With the realization that small molecules could be linked into long chains using the proper chemistry, the field of polymer chemistry exploded in the 1900s. There are two fundamental types of polymerization processes: (1) The chain-growth method sometimes known as radical polymerization and (2) step-growth polymerization. In many cases, step growth polymerization results in the elimination of water, in which case it is also known as condensation polymerization.
Chain-Growth Polymerization

Chain growth polymerization occurs largely through the coupling of double bonds in organic molecules upon the introduction of a radical - a molecule that has an unpaired electron. The organic starting material is known as the monomer, a term used generically to refer to the small molecule building blocks from which polymers are made. Polymers are also known as macromolecules. Radicals are highly reactive species and they can add to a double bond in an organic molecule to create a new radical that is centered on one of carbon atoms in the organic molecule. This radical can in turn add to another molecule with a double bond.
The process can be divided into three fundamental steps. The first is the initiation that adds the radical to the monomer and begins the process. The second step, called propagation, occurs as the new organic radical adds to additional monomer. At the end of the process, the remaining radicals are consumed in the termination step. This can happen by combination of two radicals, including the radicial initiator or some other organic radical. This is shown in the figure for the polymerization of ethylene (systematic name = ethene), which produces polyethylene, which is widely used in households as plastic wrap.
While polymerization is conceptually a simple process, it becomes more complicated as the monomers that are polymerized become more complex. A wide variety of organic monomers have been polmerized to yield useful materials based on these same principles as seen in the chart below. Additionally, multiple monomers may be polymerized together or in blocks by varying the order and timing of the addition of the monomers to the growing polymer. It should also be noted that the drying oils (e.g., linseed oil, poppyseed oil, walnut oil, etc) and other coatings also form polymers as part of their 'drying' process. Artists in the past century have increasingly created novel art objects that incorporate many of the modern materials such as collages and sculptures, and conservators must be aware of their properties in conserving those objects.
| Various Polymers, Their Properties and Uses | |||||
| Monomer | Polymer | Repeating unit | Other Names | Properties | Applications |
| ethene (ethylene), CH2=CH2 | polyethylene | -[CH2-CH2]n- | LDPE ♶ (low density polyethylene) |
soft, waxy solid | plastic film, plastic bags |
| ethene (ethylene), CH2=CH2 | polyethylene | -[CH2-CH2]n- | HDPE ♴ (high density polyethylene) |
rigid, translucent solid | electrical insulation bottles, toys |
| propene (propylene), CH2=CH(CH3) | polypropylene ♶ | -[CH(CH3)-CH2]n- | PP | atactic:* - soft, elastic isotactic:* hard, strong solid |
similar to LDPE carpet upholstery deli containers microwaveable containers |
| vinyl chloride, CH2=CHCl | poly(vinyl chloride) | -[CH2-CHCl]n- | PVC ♵ | strong, rigid solid | pipes siding flooring |
| vinylidene chloride, CH2=CCl2 | poly(vinylidene chloride) | -[CH2-CCl2]n- | Saran A | dense, high melting solid | seat covers films |
| styrene, CH2=CH(C6H5) | polystyrene | -[CH2-CH(C6H5]n- | PS ♸ | hard, rigid, clear solid soluble in organic solvents |
toys cabinets foam packaging |
| acrylonitrile, CH2=CH(CN) | polyacrylonitrile | -[CH2-CH(CN)]n- | PAN Orlon Acrilan |
high melting solid soluble in organic solvents |
rugs blankets clothing |
| tetrafluoroethylene, CF2=CF2 | polytetrafluoroethylene | -[CF2-CF2]n- | PTFE Teflon |
heat resistant, smooth solid | nonstick surfaces electrical insulation |
| methyl methacrylate, CH2=C(CH3)(CO2CH3) | poly(methyl methacrylate) | -[CH2-C(CH3)(CO2CH3)]n- | PMMA ♹ Lucite Plexiglas |
hard, transparent solid | glass substitute lighting covers signs skylights |
| vinyl acetate, CH2=CH{O-C(=O)CH3} | poly(vinyl acetate) | -[CH2-CH{O-C(=O)CH3}]n- | PVAc | soft, sticky solid | latex paints |
| isoprene, CH2=CH-C(CH3)=CH2 | cis-polyisoprene | -[CH2-CH=C(CH3)-CH2]n- | natural rubber | soft, sticky solid requires vulcanization |
automobile tires |
| chloroprene, CH2=CH-CCl=CH2 | polychloroprene (cis + trans) | -[CH2CH=CCl-CH2]n- | Neoprene | tough, rubbery solid oil resistant |
synthetic rubber |
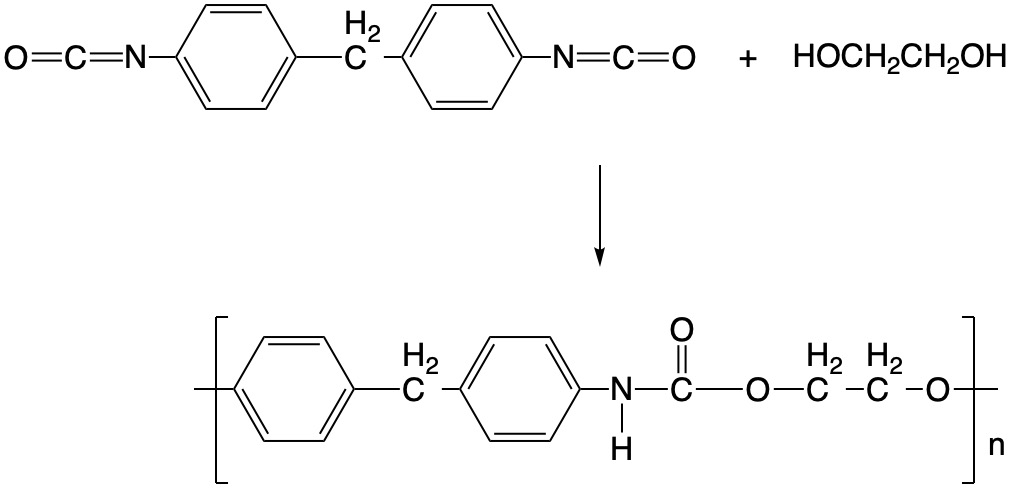
| ethylene glycol + terephthalic acid HOCH2CH2OH + HO2C-C6H4-CO2H |
poly(ethylene terephthalate) | -[O-CH2CH2-O-C(=O)-C6H4C(=O)]n- | PETE ♳ | tough, rubbery solid oil resistant |
soda bottles clothing |
| *atactic: the side CH3 groups are placed randomly; **isotactic: the side CH3 groups alternate regularly along the polymer chain | |||||
Step-Growth Polymerization
Shortcuts
Crystal Structure Data
The structures on this page were created using ChemDoodle 3D (v 6.2.1) and/or CrystalMaker X (v. 10.5.5).
Credits for the Header Image
- Album Quilt, American, c. 1840-1855, Cotton, from the Collection of the Museum of Fine Arts Houston, Public Domain.
- Day Dress and Capelet, unknown American, 1860s, Silk and Taffeta, from the Collection of the Museum of Fine Arts Houston, Public Domain.
- Hercules Slaying King Laomedon, Tapestry, Flemish (probably Tournai), 1480-1500, Wool and Silk, from the Collection of the Museum of Fine Arts Houston, Public Domain.
- Rug, Indian, 19th Century, Cotton Print, from the Collection of the Museum of Fine Arts Houston, Public Domain.













