Organic substances, just like inorganic materials, have been used for thousands of years for their desirable colors. It is pretty obvious to anyone who works with plants that they are notorious for staining cloth, and this observation was turned into a deliberate process. Thus, it is not surprising that many of these organic materials found use as dyes in addition to being harvested as pigments for painting and other applications such as cosmetics. As you explore these substances, keep in mind that the colors are highly dependent on a large variety of factors and the colors provided in the figures should be treated as approximate. As with inorganic pigments, the crystallite size of the compound will affect the perceived color. But dyeing is also affected by the pH of the dye process as well as the particular type of cloth being dyed. Dyes may require a mordant in order to make them stick to the fabric, and the mordant may also impact the color. In some cases a single dye can produce dozens of colors by simply varying the conditions under which the dyeing takes place. For an explanation of how organic pigments and dyes get their colors, please visit Pigments & Dyes. Additionally, everyone has differences in their visual sensitivity to the different colors in the visible spectrum and will not perceive things in exactly the same way.
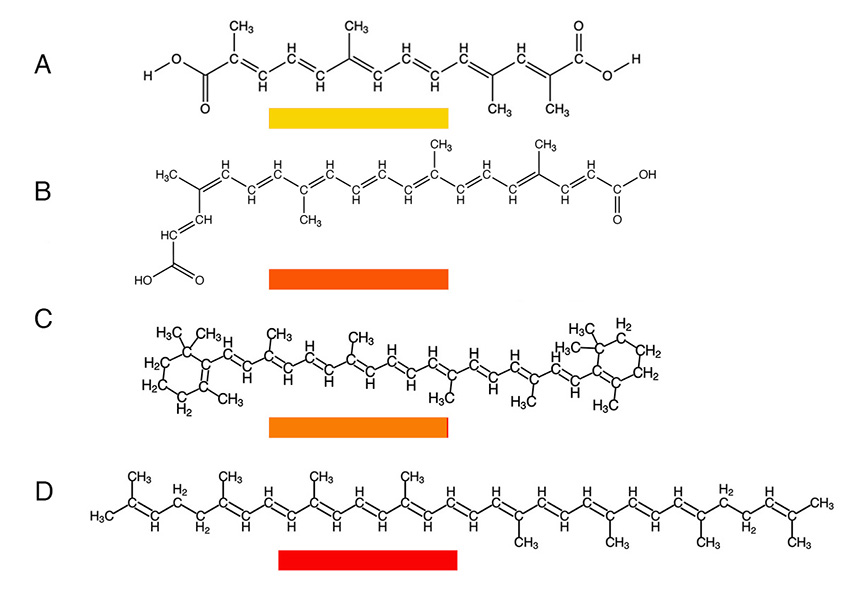
The first organic pigments to be used were those that were harvested from nature as natural plant or animal products, and they are composed largely of carbon, hydrogen, oxygen and nitrogen. Other atoms such as phosphorus or sulfur may also be present. Atoms other than carbon and hydrogen are referred to as heteroatoms. Many organic compounds are colorless, but some bonding situations will allow these molecules to absorb light in the visible region of the electromagnetic radiation spectrum. See a more detailed discussion of that process at the bottom of this page. The common feature they all possess is a number of multiple bonds adjacent to other. For example, β-carotene is an orange molecule that gives carrots their characteristic orange color. Saffron, annatto and lycopene have similar structures. Lycopene produces the red color in tomatoes, while saffron is a yellow compound derived from yellow color of crocus stamens. Lycopene and β-carotene are isomers - they have the same chemical formula but different structures, as seen in the figure.

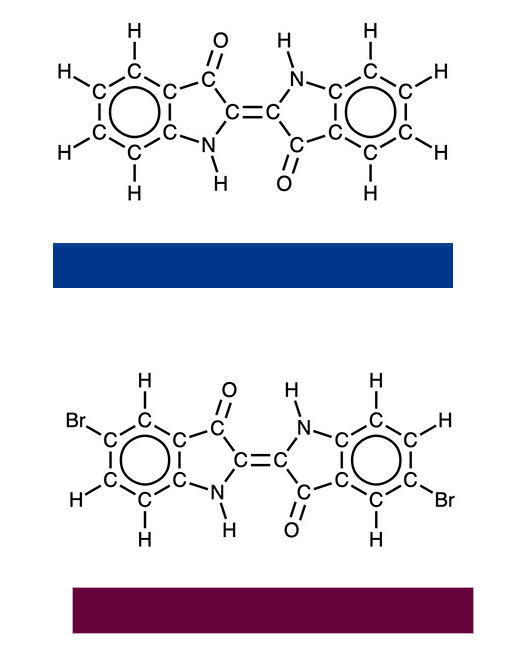
Other important natural organic dyes include indigo, which was harvested primarily from plants in Indigofera tinctoria from subtropical areas in Asia. Strobilanthes cusia from Japan and Taiwan or woad in Europe are also sources of natural indigo. Indigo is used for the blue color in blue jeans. The structure of Tyrian purple is the same as that off indigo with a couple of hydrogen atoms replaced with bromine. Tyrian purple was harvested from sea snails in a laborious and expensive process. As a result it was only affordable by the rich and its use was largely restricted for garments of the wealthiest classes and was, consequently, also known as royal purple.
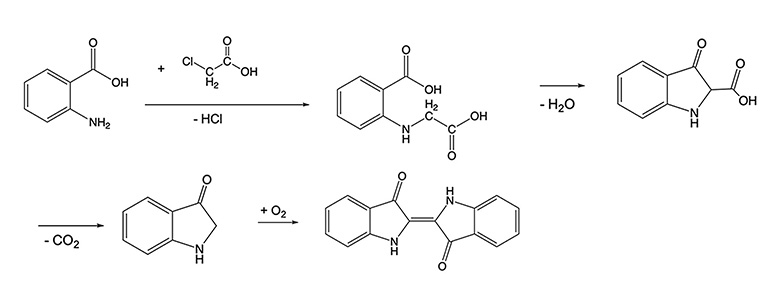
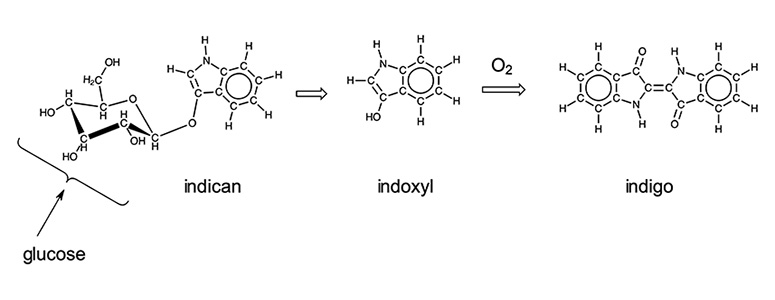
Indigo can be synthesized from common organic molecules as shown below, and many organic dyes have now been prepared in laboratories and chemical plants. An entire industry devoted to the development of organic pigments and dyes has developed as a result. The dye that is extracted from plants, indican, is a precursor to the indigo molecule. As can be seen in the scheme below, half of the indigo molecule is bonded to a glucose molecule. In the process of dyeing, the glucose is cleaved and the result molecule dimerizes upon reaction with oxygen in the air. When first placed in an indigo dye vat, yarn will take on a yellow to green color that then turns blue as oxygen reacts with the pigment. There are numerous Youtube videos that show this process in action.
Lawsone is one of many quinone-based dye molecules. The quninone structure is a simple six-membered benzene ring that has oxygen atoms attached at opposite ends of the molecule (see figure). It is important in chemistry and biology because it undergoes reversible reduction-oxidation processes with hydroquinone. Additional aromatic rings can be added to for napthoquinone and anthraquinone, whose names derive from the hydrocarbons naphthalene and anthracene. Lawsone is extracted from the henna plant, Lawonia enermis and produces a tan to dark brown color depending on the dyeing conditions. It has been used as a skin dye for thousands of years.
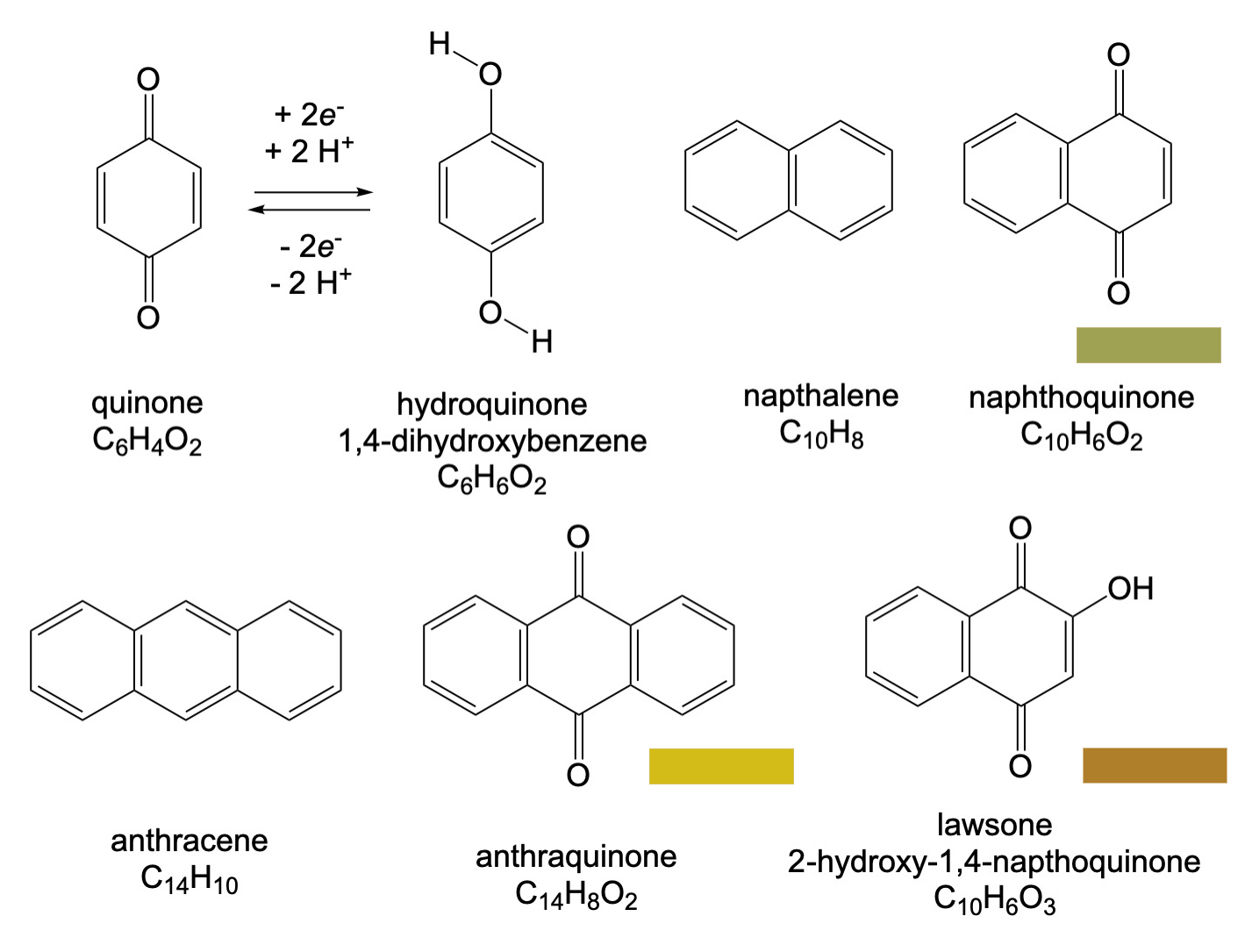

Alizarin is also a quinone-based pigment. It is red dye that has been used since at least 1500BC. It and the structurally similar purpurin molecule, are obtained from the roots of the madder plant. They are derivatives of anthraquinone, from which a number of synthetic dyes are also based. Syntheses of the dyes were developed in the 1800s and alizarin is commercially prepared by BASF starting with anthracene, C14H10. In paints it is known as alizarin crimson or Rose madder.
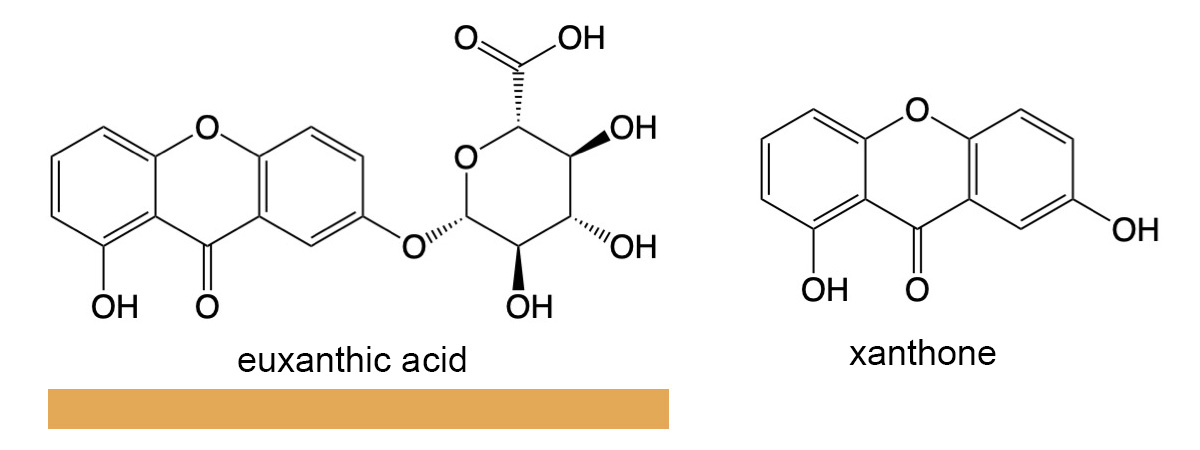
Indian yellow was used from the 1500s through the 19th century as a popular pigment, especially in Indian miniature paintings during that time period. Its method of production was unusual by modern standards. The pigment was harvested from the urine of cows that were fed exclusively mango leaves. Its usage was curtailed in the 20th century because the exclusive mango leaf diet was deemed harmful to the health of the cows. Indian yellow is a mixture of magnesium and calcium salts of euxantic acid, C19H16O10, xanthone (a chemical precursor to euxanthic acid) and sulfonated xanthone.
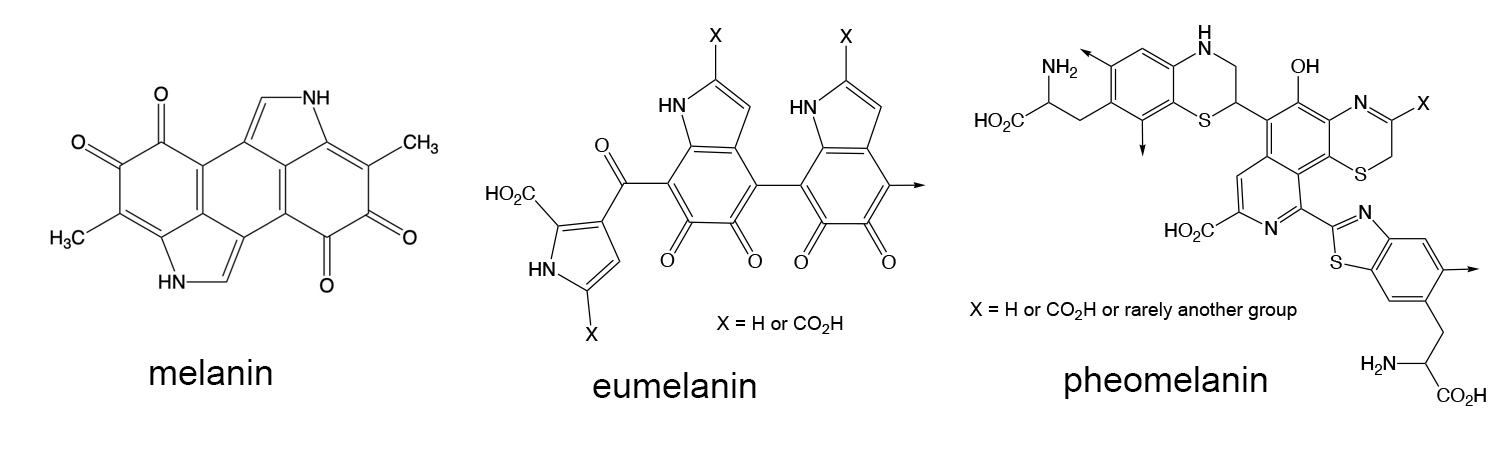
Melanin and related compounds. Melanin is the molecule responsible for skin color and acts to prevent damage from UV radiation. The basic structure of melanin undergoes transformation into two general polymeric structures in the the human body - eumelanin and pheomelanin. These polymers have some variation in their structures depending on the nature of the various subtituents attached to the polymer. Eumelanin can be either black or brown, and pheomelanin is yellow-red in color. Together these polymers are responsible for hair color. As far as Art is concerned, melanins are also a major component of the protective ink of cephalapods such as squid and are found in both brown (sepia) and blue-black colors. These natural melanins are harvested and used as inks
Synthetic Organic Pigments and Dyes
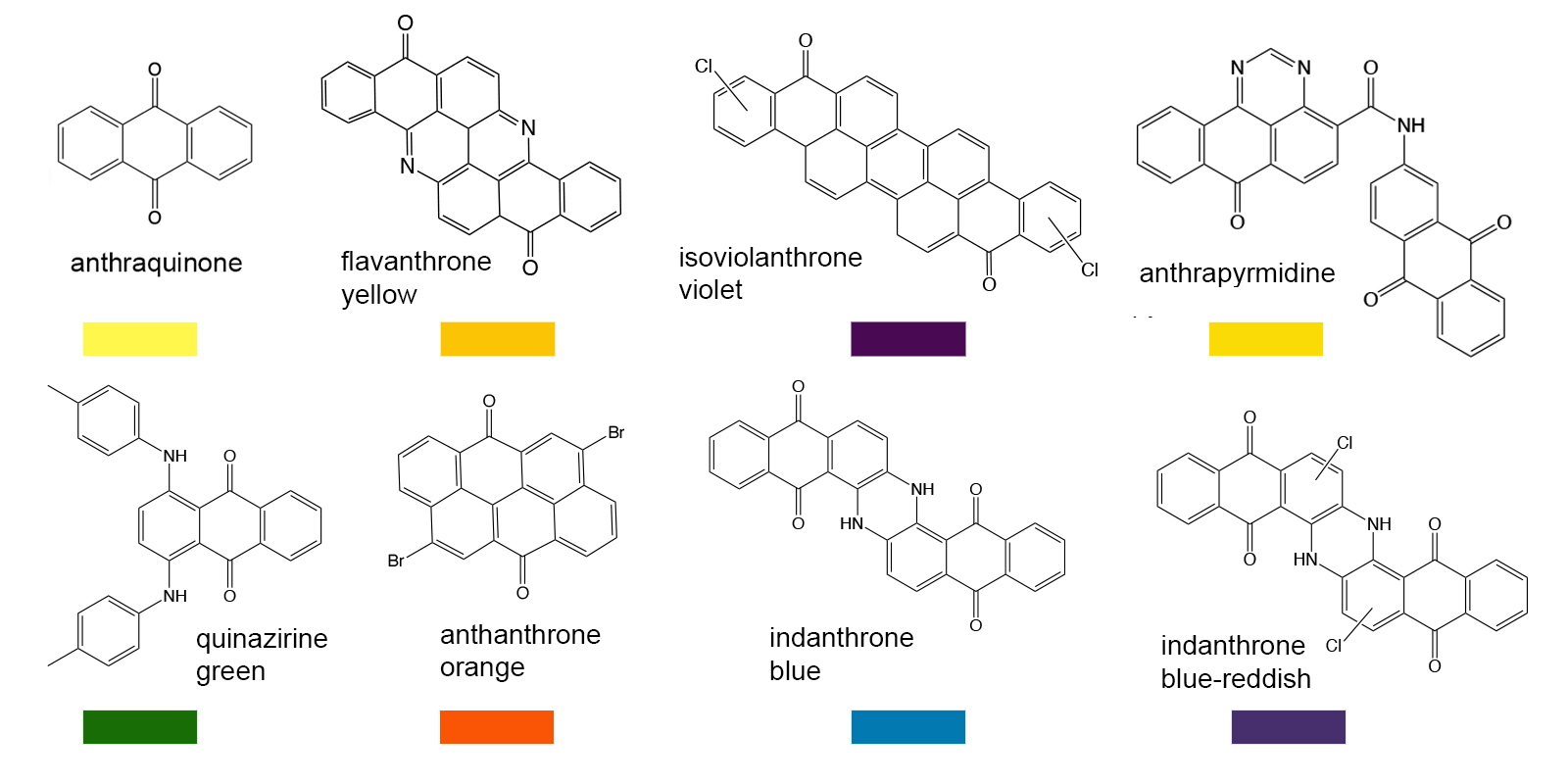
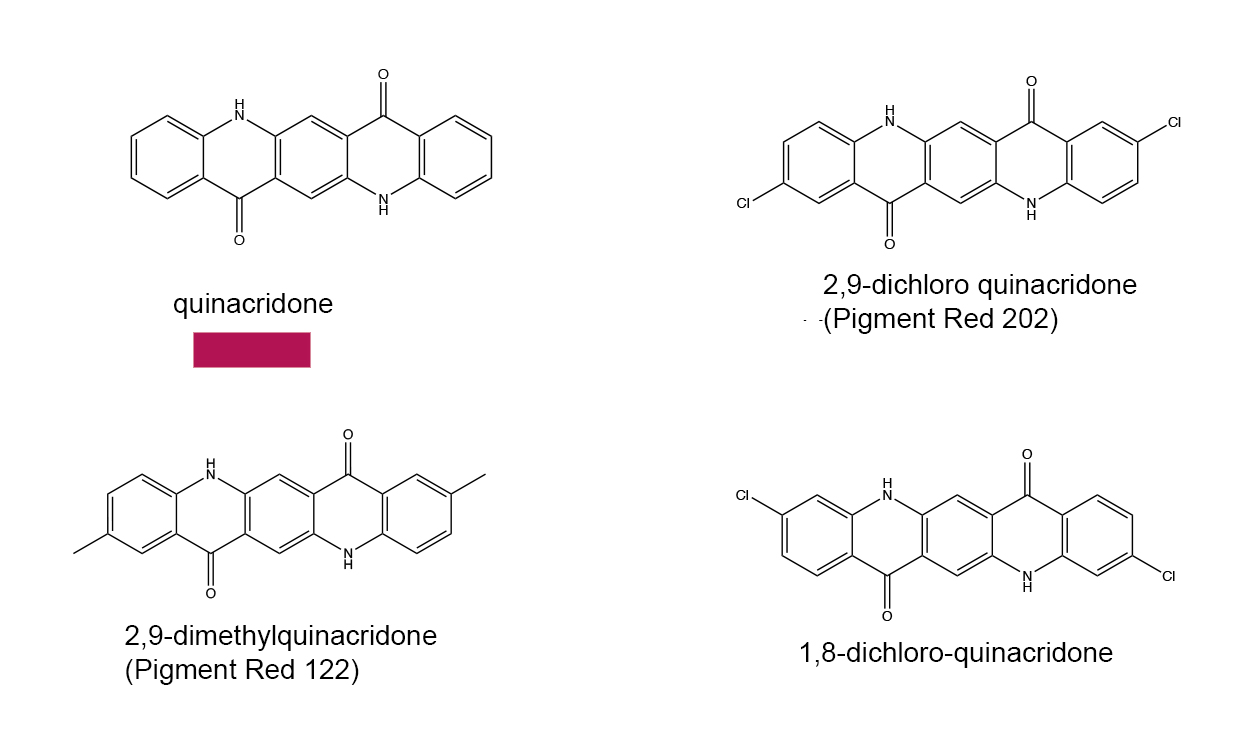
Based on the growing understanding of chromophores and the effects that different functional groups had on their colors, chemists made rapid advances in the laboratory synthesis of a wide variety of new organic compounds for use as pigments and dyes. Not only were paints and textile dyes the intended market, but colorants also found applications in inks, hair dyes, food dyes, additives to cosmetics and so on. There are too many synthetic dye molecules to list them all here, but a few classes will be noted to illustrate the common chromophores. The core chromophores can be altered to produce a wide variety of colors.
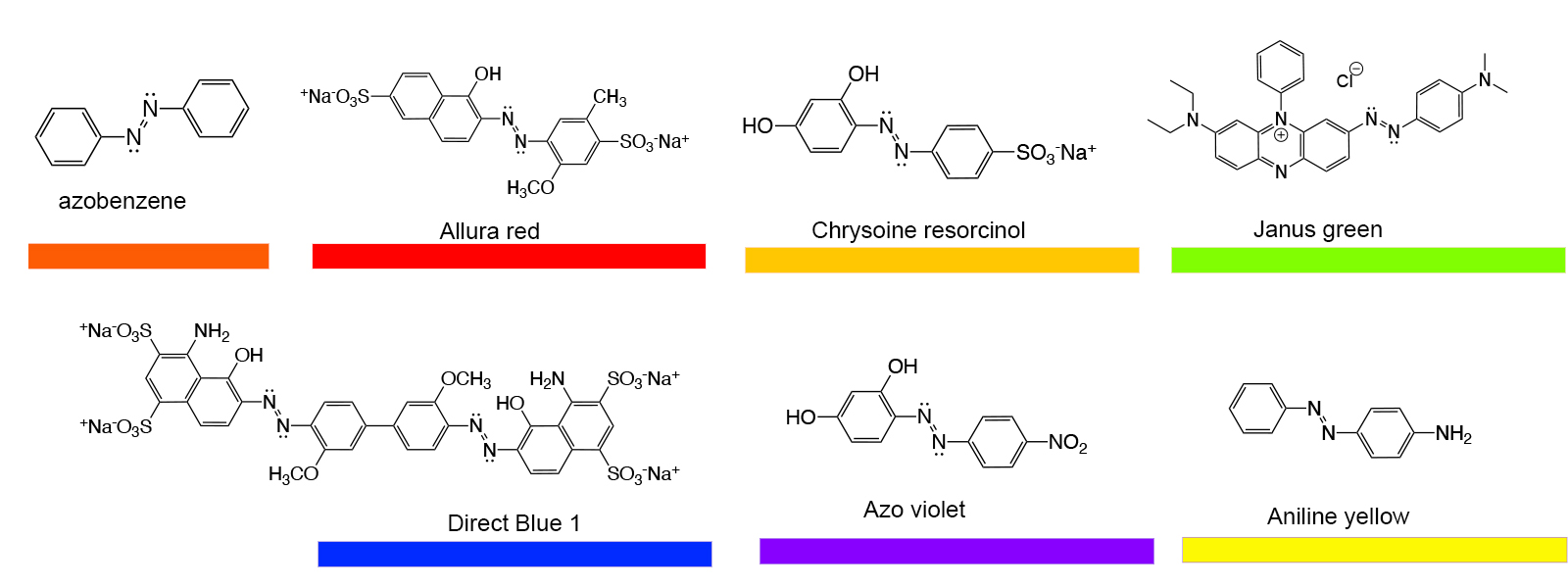
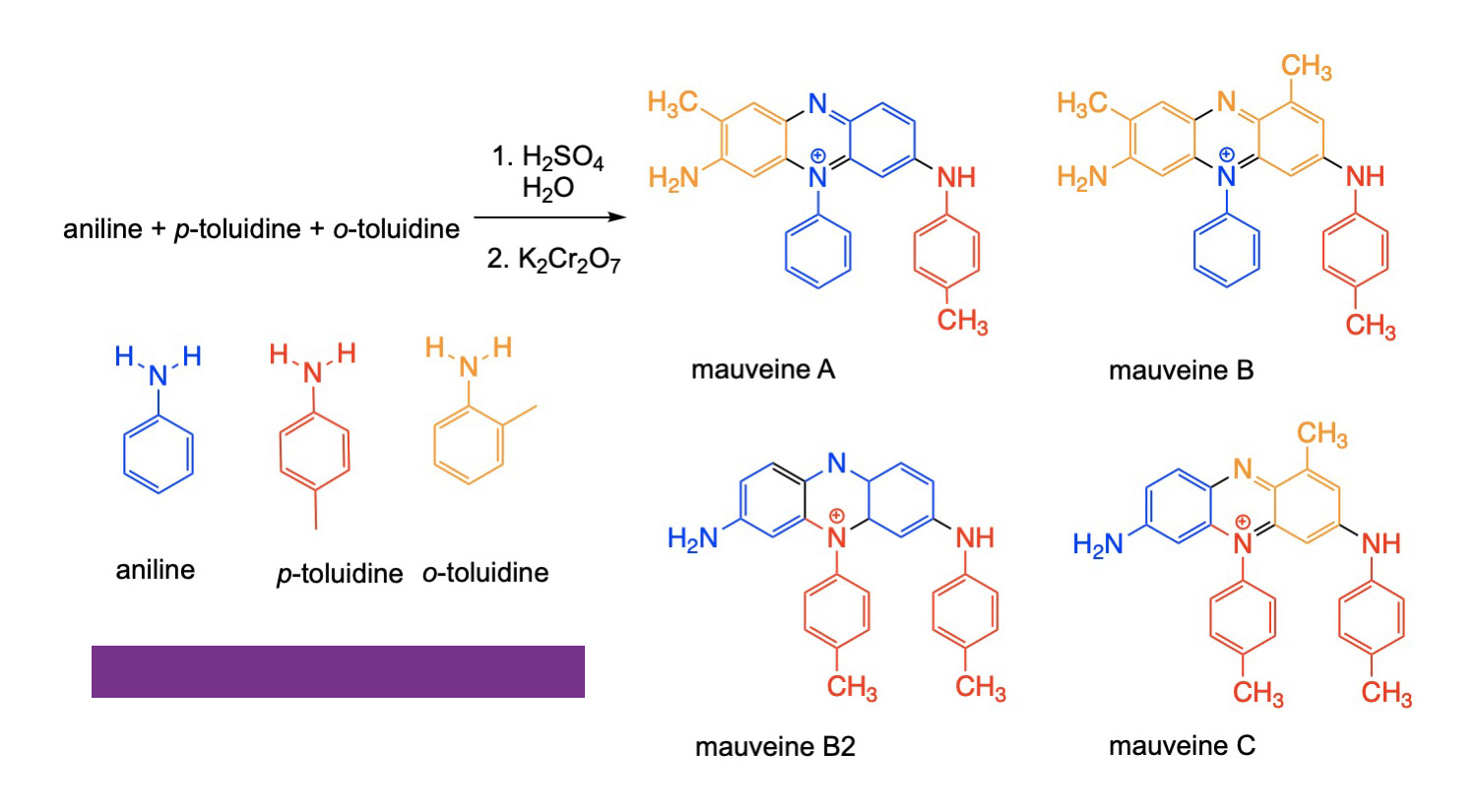
William Henry Perkins discovered the aniline dyes in 1856. As suggested by the name, these are based on aniline, a benzene molecule with an NH2 group replacing a hydrogen, and its derivatives. It was sometimes called phenylamine. When reacted with the methyl-substituted anilines p-toluidine and o-toluidine, a mixture of isomers results that have a purple color. Some of the isomers are shown in the figure. Given that aniline, p-toulidine and o-toulidine are very similar molecules except for the presence of a methyl group, it is not surprising that the coupling of four of these molecules could occur with any of the three in any of the four possible positions in the final product. Conceivably one could get up to 81 different compounds, so it is not surprising that the actual pigment is a mixture of several compounds. The compound without methyl groups attached is known as pseudo-mauveine, and no crystal structure was reported for any of the possible products until 2015, although spectroscopic characterization such as nuclear magnetic resonance spectroscopy (NMR)had confirmed the structures of the major compounds in the pigment mixture. Originally known as aniline purple, the name was changed to mauveine based on the French name for the mallow flower for marketing purposes. Workers in the aniline dye industry were found to have an increased risk of bladder cancer, and so these compounds have fallen from favor.
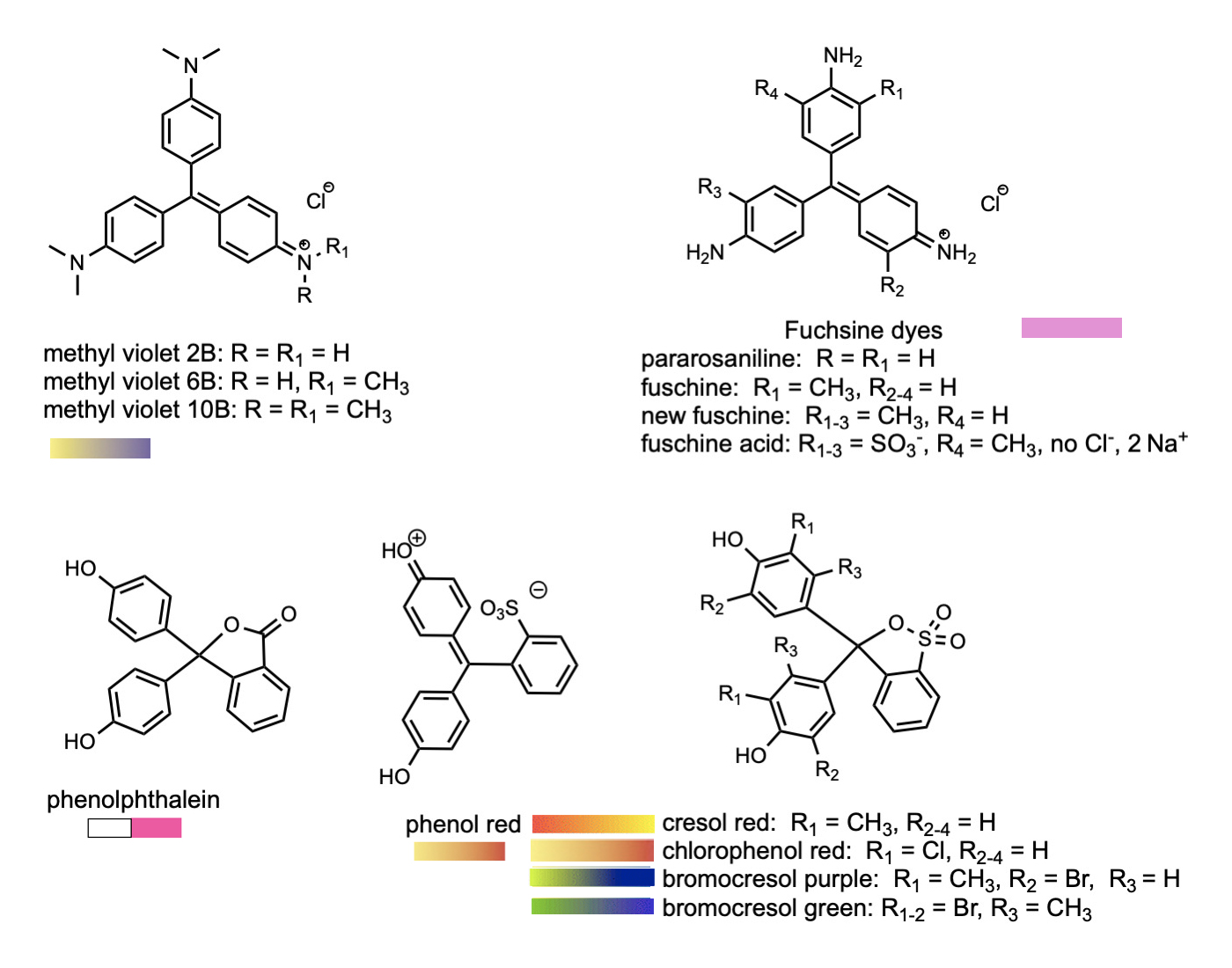
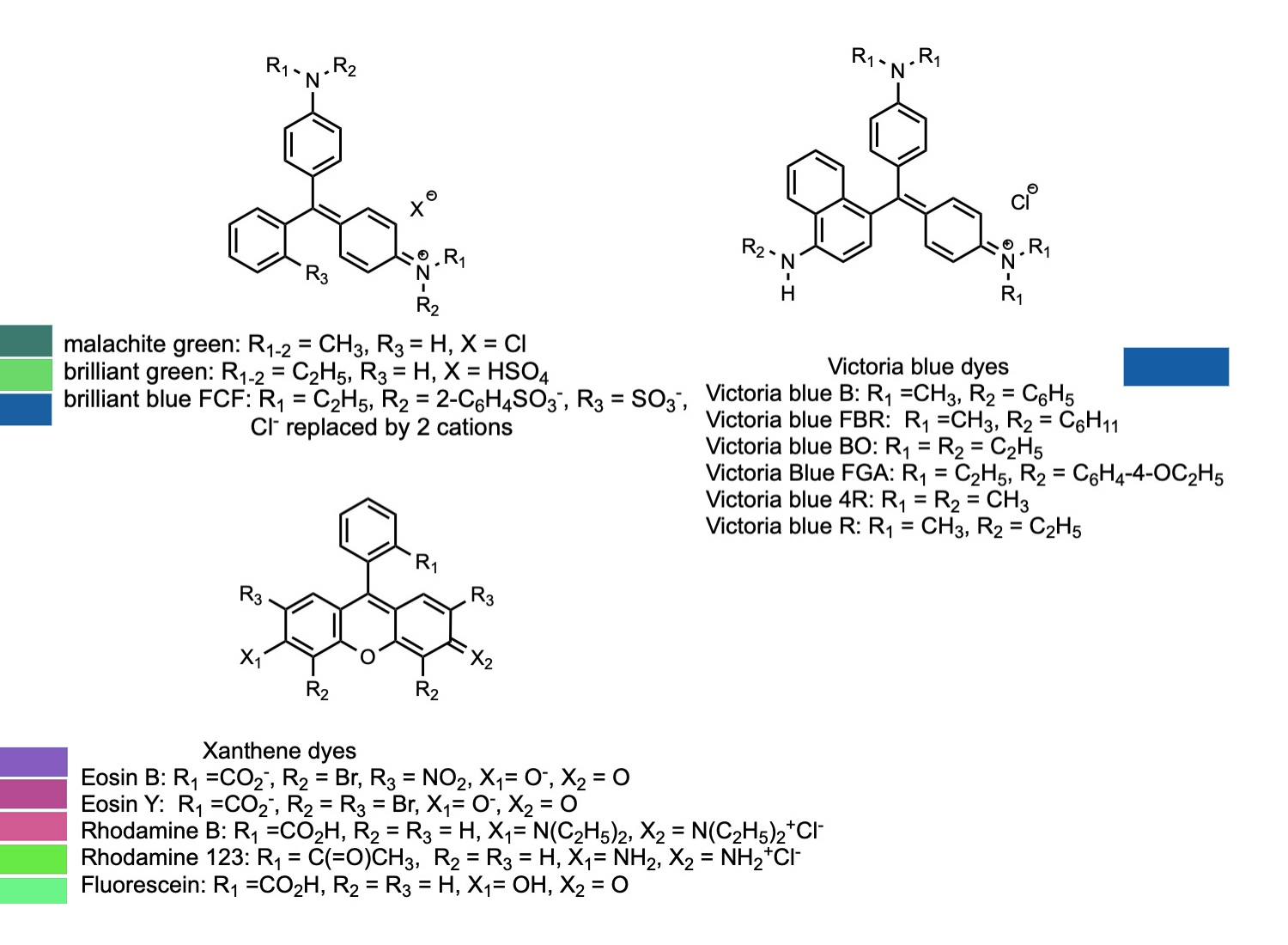
Another class of common dyes is base on trphenyl methane, HC(C6H5)3. Phenyl is the name given to the benzene molecule when it has one of the hydrogen atoms and is attached to a different functional goup. More generally, the phenyl groups can be a wide variety of aromatic ring systems like benzene. The general term for such groups as attached to another organic molecule is aryl group. A number of these compounds are indicators that are used to detect pH of solutions as the colors of the compounds change as the pH changes. The most widely known of these if phenolphthalein, which changes from colorless in acidic solution to bright pink in the presence of base. Others of these such as eosin and rhodamine are used in biology to stain different types of tissues to make them show up better when viewed by a microscope. An added bonus is that some of these dyes are fluorescent, which makes them show up very brightly when irradiated by the proper wavelength of light.
Identification of Organic Pigments
Infrared and Raman spectroscopy are a good methods for identification of organic pigments. These techniques use the vibrational excitations in the organic molecule as a fingerprint for determining the functional groups that are present, such as an azo or carbonyl group. An example can be found at "Identification of Synthetic Organic Pigments in Contemporary Artists’ Paints by FT-IR and FT-Raman: An Advanced Analytical Experiment," M. Longoni and S. Bruni, J. Chem. Educ. 2021, 98, 966–972. Often this information is coupled with X-ray fluoresence, which is a technique used to identify the presence and identity of metallic elements. Of course, an organic molecule would not have a metallic element present, which would be an indication that the pigment must be organic organic. In some cases, X-ray diffraction may also be useful for identifying organic pigments (see "Combined X-ray Diffraction and Raman Identification of Synthetic Organic Pigments in Works of Art: From Powder Samples to Artists' Paints" , L. Brostoff, S.A. Centeno, P. Ropret, P. Bythrow, F. Pottier, Anal. Chem. 2009, 81, 6096–6106; https://doi.org/10.1021/ac9004953. A more in-depth discussion of these techniques is available on the page Analytical Methods.
Shortcuts
Crystal Structure Data
- Structures for which crystallographic information format (CIF) were available were obtained from the Cambridge Crystallographic Data Centre. Those not in the database were created using ChemDoodle 3D and/or CrystalMaker.
- Aliazrin yellow: Yatsenko A V, Paseshnichenko K A, CCDC 996074 , 2014, DOI: 10.5517/cc12fhff
- Alizarin, 1,2-dihydroxy-9,10-anthraquinone: Cyranski M K, Jamroz M H, Rygula A, Dobrowolski J C, Dobrzycki L, Baranska M, CrystEngComm, 2012, 14, 3667, DOI: 10.1039/c2ce06063a
- Carmine red, potassium salt: Ishida T, Inoue M, Baba K, Kozawa M, Inoue K, Inouye H, Acta Crystallographica C, 1987, 43, 1541, DOI: 10.1107/S0108270187091169
- Mauveine: Plater M J, Harrison W T A, Rzepa H S, , J. Chem. Res., 2015, 39, 711-718, DOI: 10.3184/174751915X14474318419130
- Indigo: von Eller H., Bulletin de la Societe Chimique de France, 1955, 1433.
- Tyrian purple: Susse P, Krampe C., Naturwissenschaften, 1979, 66, 110, DOI: 10.1007/BF00373504
Department of Chemistry

Houston, TX
6100 Main St., Houston, TX 77005-1827 | Mailing Address: P.O. Box 1892, Houston, TX 77251-1892 713-348-0000 |












