Diamond is a naturally-occurring form of carbon in which every carbon atom is bound to four other carbon atoms in a tetrahedral array with covalent single bonds. This three-dimensional structure makes diamond extremely hard. Diamonds are formed in the earth under extreme temperatures and pressures, and they are forced to the surface during periods of volcanic activity. Diamonds can also be made synthetically at high temperatures and pressures. They possesses a face-centered cubic (FCC) crystal lattice. Normally diamonds are colorless, clear stones but they can adopt a variety of colors (see below). Diamond also has a high optical dispersion, which means that the crystal lattice affects the velocity of light of different frequencies passing through it differently. The result is the highly lustrous quality of cut diamonds. Lonsdaleite is also known as hexagonal diamond and was first discovered in 1967. It is interesting to compare its structure to that of graphite (see below). While they are both hexagonal, lonsdaleite has covalent bonds between the layers of carbon atoms.
In the interactive structures, dragging the mouse on the image will cause the structure to rotate, while holding down the shift key while dragging will allow the structure to be resized, and the same holding the option key will rotate the structure in the plane of the screen.
Different forms of the same chemical element are known as allotropes. In addition to diamond, lonsdaleite, graphite and graphene, carbon has several other allotropes including the fullerenes such as C60, whose discovery in 1985 led to 1996 Nobel Prize in Chemistry awarded to Harry W. Kroto, Richard E. Smalley and Robert F. Curl. Smalley and Curl were on faculty at Rice at the time of the discovery. An interactive model of C60 and its crystal lattice may be found here.
Because of the cost of real diamonds, substitutes have been sought. Glass has been cut and shaped to resemble diamonds, but its properties are very different and so a reflective coating is placed on the bottom to make the imitation glass reflect light similar to diamond. The density of diamond is ca. 3.5 g/cm3 while ordinary glass as about 2.5 g/cm3, while leaded glass is about 3.1 g/cm3. The hardness of glass is substantially lower, at around 5.5 - 7 on the Mohs scale. Another material that has been used for imitation diamonds is colorless cubic zirconia, ZrO2. As the name implies, the crystal structure is cubic like diamond but, in contrast, it does not cleave easily and is brittle. The structure can be described as a face-centered cubic arrangement of zirconium atoms with the oxygen atoms occupying all of the tetrahedral holes in that FCC lattice (see the interactive structure). This makes each zirconium atom eight coordinate - meaning that they are each bonded to eight oxygen atoms. While diamond also forms an FCC arrangement of carbon atoms, only half of the tetrahedral holes are occupied by carbon atoms, which is due to carbon only needing to form four bonds to other atoms. Cubic zirconia is more dense than diamond (5.6 to 6 g/cm3 but has a hardness of 8.0 to 8.5 Mohs. It has a refractive index of about 2.15, while that for diamond is around 2.42. Because of its high melting point (2750 °C), crystals of cubic zirconia are challenging to prepare. In order to stabilize it in the cubic crystal lattice during synthesis, yttrium oxide, Y2O3 is added. In addition to the greater density, cubic zirconia can be distinguished from diamond by its fluorescence under short-wave ultraviolet light.
Like diamond, graphite is a naturally-occurring form of carbon but in graphite each carbon atom is bound to only three other carbon atoms by covalent bonds. In order to satisfy the bonding needs of each carbon atom, there is also some multiple bonding between the carbon atoms. The network of multiple bonds is responsible for graphite being a good electrical conductor. Another consequence of the multiple bonding network is that graphite readily absorbs light, which makes it black, unlike diamond whose three-dimensional single-bonded network results in a colorless substance in the absence of impurities and defects.
This bonding structure causes the formation of stacks of two-dimensional sheets of carbon atoms that are are only weakly bound to each other, and the sheets can slip easily over each other making graphite a good lubricant. These layers are so weakly held together that they can be separated simply by putting a piece of transparent tape on graphite and pulling it off. Andre Geim and Kostya Novoselov received the Nobel Prize in Physics in 2010 for this very simple discovery. The resulting single layer of the graphite lattice is known as graphene, a substance that has very interesting electrical and strength properties that are being widely studied for applications in materials science.
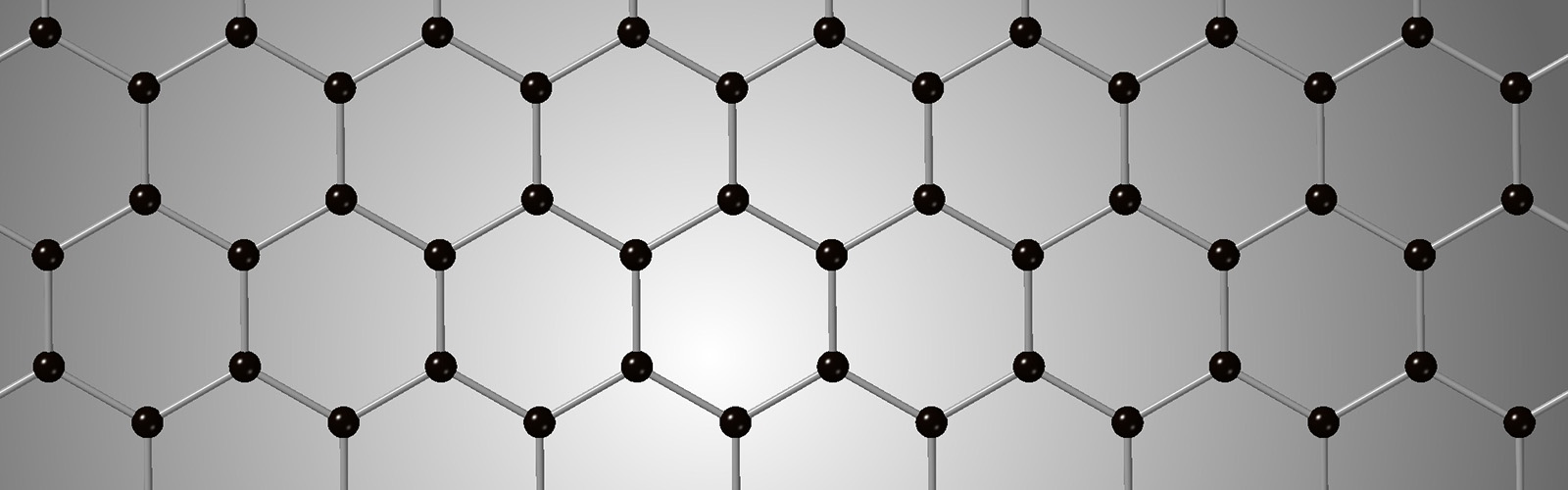
Graphene
Color in diamonds

Diamonds may exhibit a variety of colors, and their color rises from defects in the crystal lattice. One type of defect is the replacement of a small percentage of the carbon atoms with another atom of similar size. Diamonds containing up to 1% nitrogen are classified as Type I. If nitrogen atoms are isolated and spread throughout the diamond lattice (Type Ib), the color of the diamond will be yellow to brown depending on the concentration of nitrogen atoms. On the other hand, if the nitrogen is found as pairs of nitrogen atoms or larger aggregates (Type Ia), the color of the diamond is not affected. 98% of Type I diamonds are Type 1a. This is because nitrogen has one more electron than carbon and these extra electrons are delocalized over the crystal if the nitrogen atoms are isolated, but will not be if two or more nitrogen atoms are located next to each other. If boron is placed in the lattice in place of carbon, the diamonds will be blue (Type IIb). One of the most famous blue diamonds is the Hope Diamond now housed in the Smithsonian Institution. Diamonds can also be pink, red or brown because of defects in the arrangements of the carbons atoms that occurred during the crystal growth process (Type IIa). Diamonds can develop color under high energy radiation. These can be red, green, blue, yellow or black. This is also observed for some diamonds that grew in close contact with radioactive minerals. The color of diamonds produced in this way will disappear if the diamonds are heated, as it allows the crystal lattice to reorganize to eliminate the structural changes that arose when the sample was irradiated.
Similar to diamond, cubic zirconia can be doped by a wide variety of metal ions that substitute for some of the zirconium atoms, producing a range of different colors: chromium (green), cobalt (blue-violet), copper (aqua or yellow), erbium (pink), iron (yellow), manganese (brown to violet), neodymium (purple), nickel (yellow to brown), praseodymium (amber), vanadium (green), cerium (red, orange or yellow), etc. The possible substitutions are not as extensive in diamond because only atoms similar in size to carbon can substitute for it (i.e., boron and nitrogen).
Shortcuts
Credits for the Header Image
- The Cullinan Diamonds I and II.
- The Hope Diamond, National Natural History Museum, Smithsonian Institution..
- The uncut Cullinan Diamond.
Department of Chemistry

Houston, TX
6100 Main St., Houston, TX 77005-1827 | Mailing Address: P.O. Box 1892, Houston, TX 77251-1892 713-348-0000 |












